Method for removing recombinant protein endotoxin by GEM
A recombinant protein and endotoxin technology, applied in biochemical equipment and methods, enzymes, hydrolases, etc., can solve problems such as destroying protein activity, and achieve the effect of reducing the loss of protein activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Construction and expression of L-asparaginase-ACMA
[0024] 1) Vector construction
[0025] Using the Escherichia coli AS 1.357 genome as a template, the L-asparaginase gene was amplified and connected to the plasmid pET28a-AcmA to obtain the recombinant expression plasmid pET28a-LA-AcmA, which was transformed into the Escherichia coli expression vector BL21 by chemical transformation.
[0026] 2) Induced expression
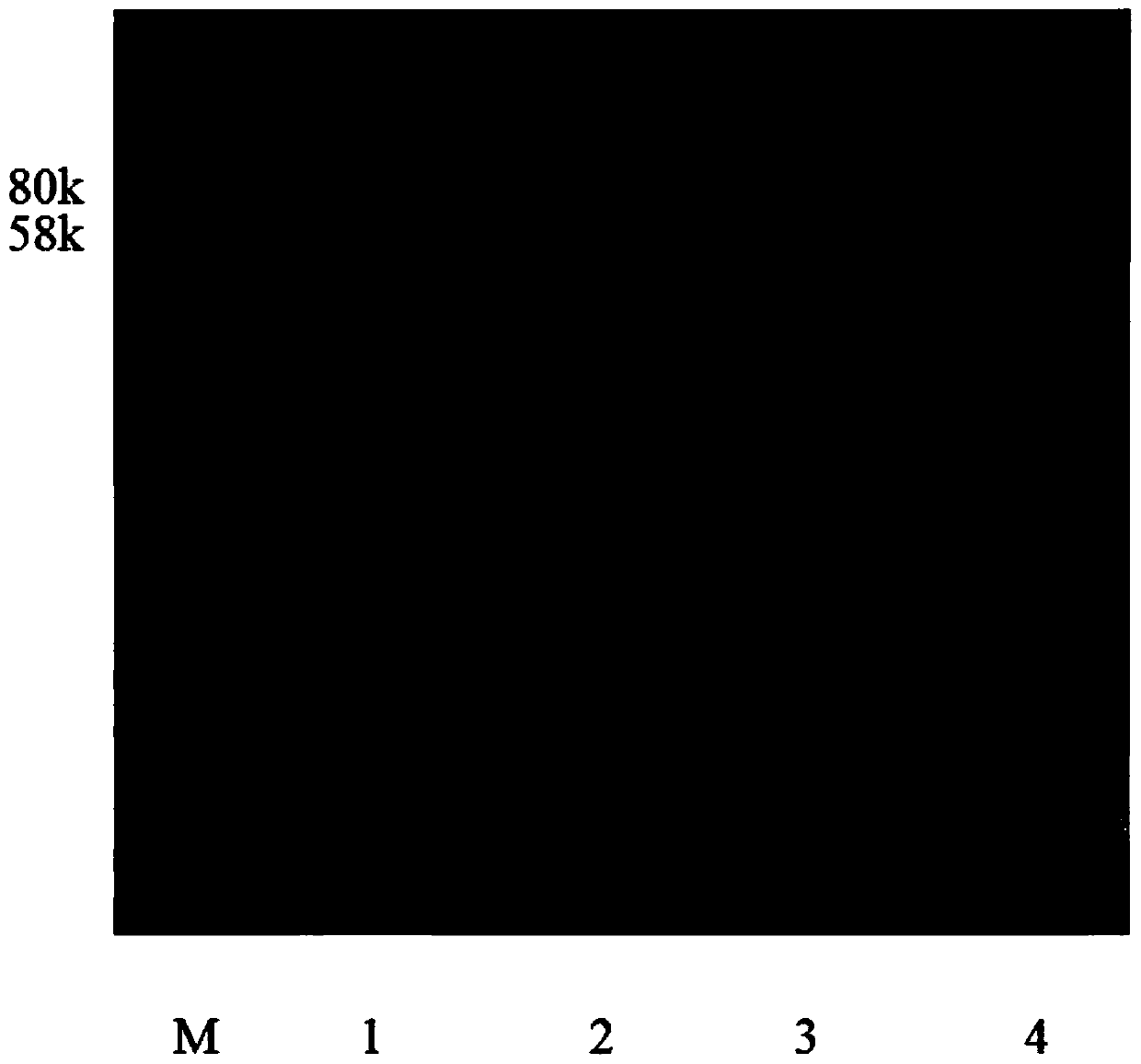
[0027] Isolate by streaking to obtain a single clone of BL21 carrying the plasmid, culture overnight to obtain a seed solution, inoculate with an inoculum amount of 1%-5%, when the OD of the bacterial solution 600 When it reaches 0.5-0.8, add IPTG for induction, the induction concentration is 0.1mM-1mM, the induction temperature is 25°C-37°C, the induction time is 12 hours-24 hours, and all media are supplemented with 50μg / mL kanamycin LB medium. Using SDS-PAGE to analyze the recombinant expression, the results are as follows figure 1 As ...
Embodiment 2
[0028] Embodiment 2: GEM purification immobilized recombinant L-asparaginase
[0029] 1) GEM preparation
[0030] Cultivate Lactococcus lactis NZ9000 to OD in M17 medium containing 1% glucose 600 Between 2.0-2.5, collect the bacteria by centrifugation at 4000rpm, wash with PBS to remove the medium, resuspend the bacteria in 0.1M-0.2M HCl, boil for 30-60 minutes, collect the precipitate by centrifugation at 8000rpm, use 50mM Tri- HCl (pH 7.2) was washed thoroughly to obtain GEM.
[0031] 2) GEM purification of immobilized recombinant L-asparaginase-AcmA
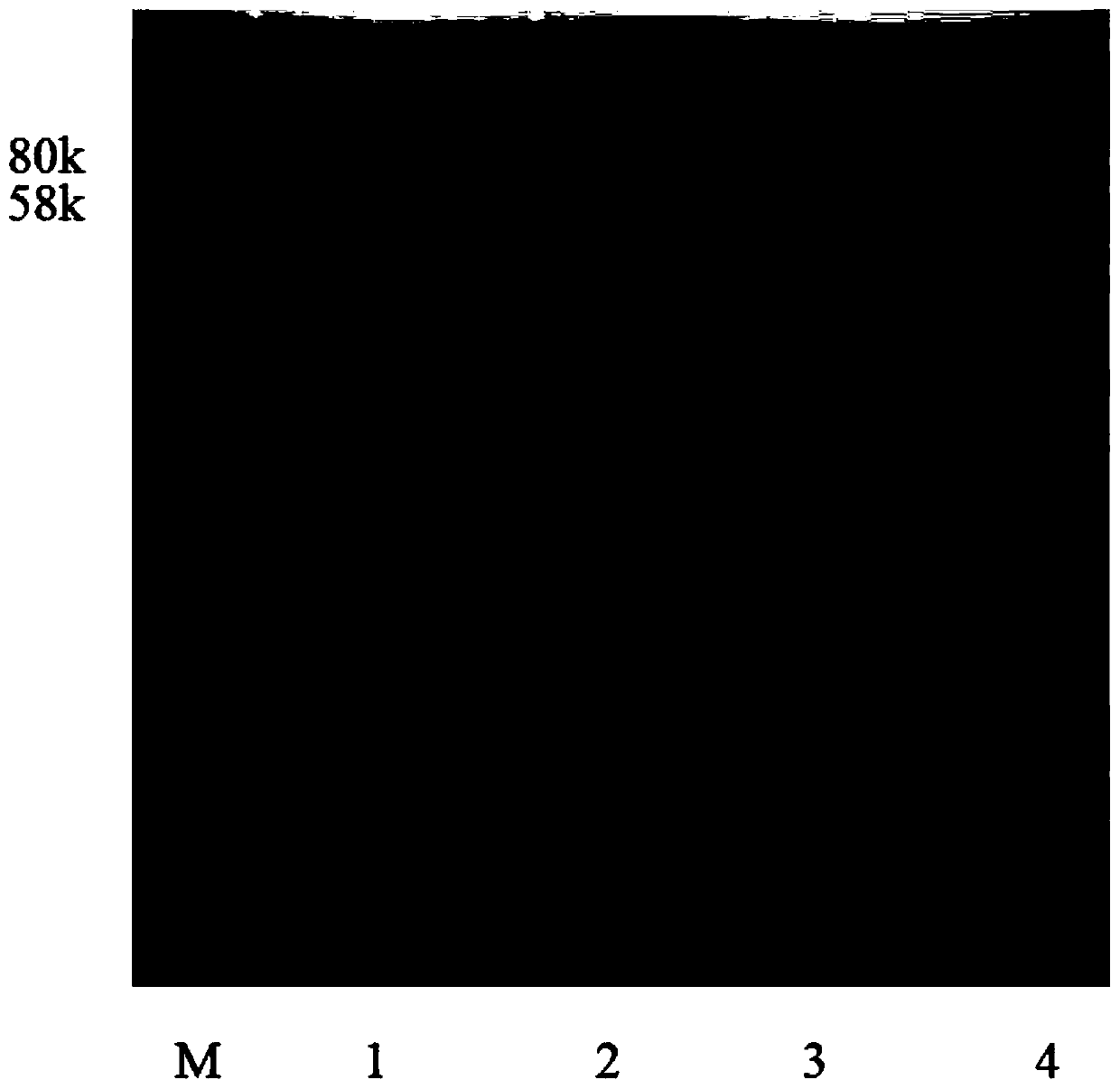
[0032] Collect the induced bacteria by centrifugation at 4000rpm, wash and remove the culture medium with pre-cooled 50mM Tri-HCl (pH7.2), resuspend the bacteria in pre-cooled 50mM Tri-HCl (pH7.2), and sonicate Cells, sonicate for 2 seconds, pause for 4 seconds, sonicate for 20 minutes, centrifuge at 8000rmp for 20 minutes to separate, take the supernatant and mix with the GEM prepared in step 3), and combine with magnetic ...
Embodiment 3
[0034] Example 3: Endotoxin removal
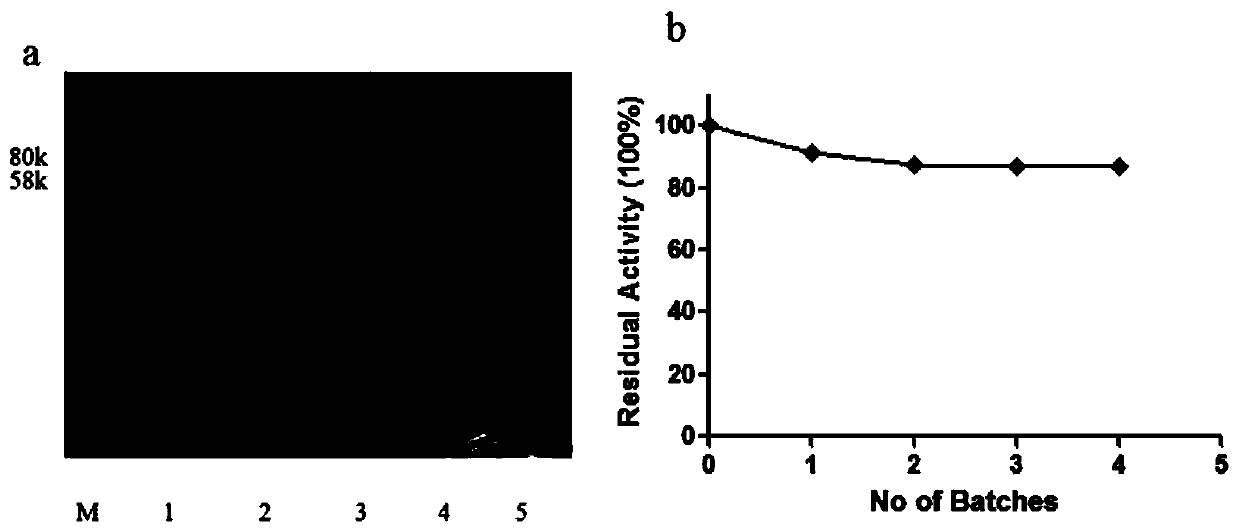
[0035] The immobilized recombinant L-asparaginase-ACMA was resuspended in 1% Triton X-114 (4°C), combined on ice for 30 minutes, centrifuged at 8000 rpm for 10 minutes (4°C), the supernatant was discarded, and washed four times. Using SDS-PAGE for analysis, the results are as follows image 3 as shown in a. Determination of the enzyme activity after four rounds of removal, the results are as follows image 3 As shown in b, more than 80% of the relative enzyme activity was still preserved after four rounds of clearance work.
[0036] In this embodiment, 1% Triton X-114 is used to remove endotoxin at low temperature (4° C.), which can guarantee protease activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com