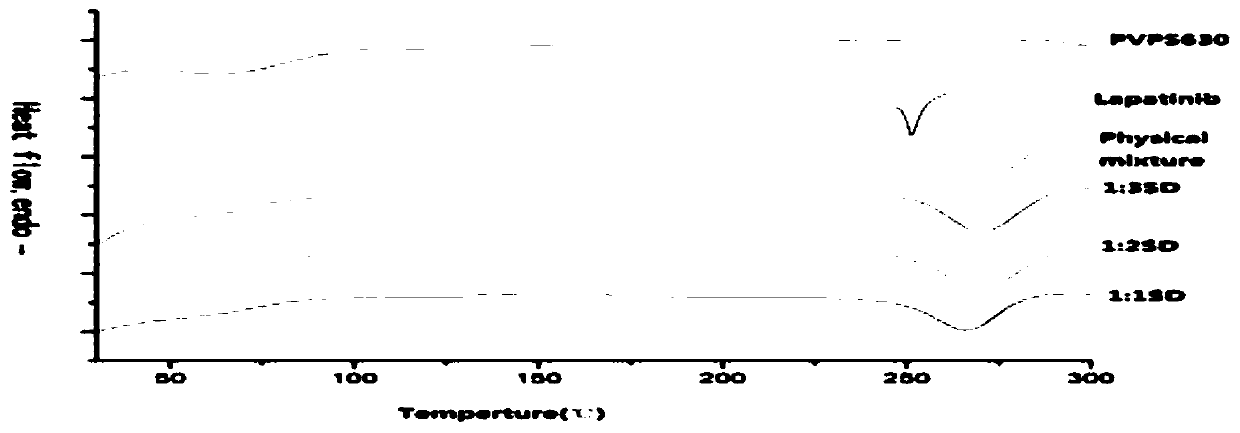
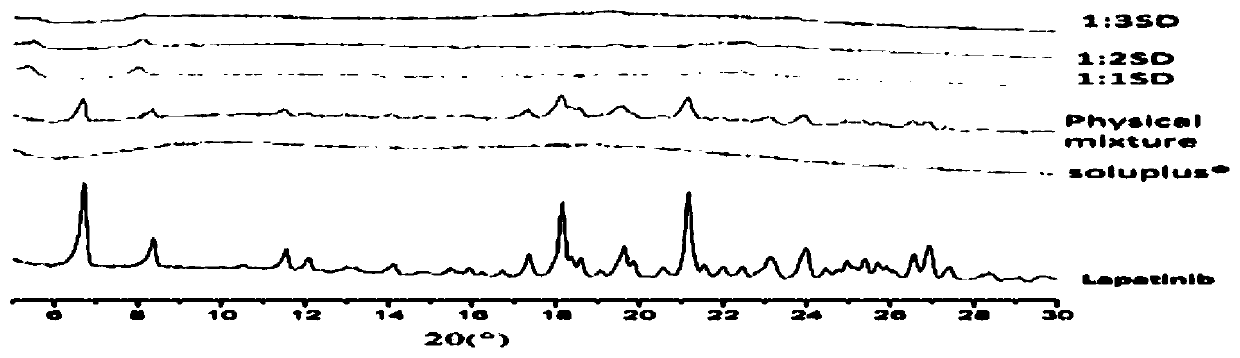
Method for preparing lapatinib ditosylate solid dispersion by freeze-drying method
A technology of solid dispersion and toluenesulfonic acid, which is applied in freeze-drying transportation, pharmaceutical formulations, organic active ingredients, etc., can solve the problem of lack of bioavailability of lapatinib toluenesulfonate solid dispersion and improve bioavailability The effect of high density, large surface area and loose packing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of freeze-drying method of the present invention prepares the method for lapatinib tosylate solid dispersion, comprises the following steps: (1) the carrier PVPS630 is macromolecular material, carries out drug loading ratio preparation physical mixture; Described drug loading The ratio is 1:1. (2) Take the physical mixture (including Lapatinib ditosylate), add it to 30mL acetonitrile solution, and ultrasonically dissolve it completely. The solution is quickly frozen in liquid nitrogen, dried in a freeze dryer for 30 hours, taken out and placed in P 2 o 5 Put in a desiccator for 30 hours, pass through a 60-mesh sieve for subsequent use, and obtain a solid dispersion of lapatinib tosylate. The weight-to-volume ratio of the physical mixture to the acetonitrile solution is 500 mg:30 mL. The volume ratio of acetonitrile and water in the acetonitrile solution is 2:1. The temperature in the freeze dryer is -40°C, and the temperature in the cold well is -60°C.
Embodiment 2
[0032] The difference between embodiment 2 and embodiment 1 is: a kind of freeze-drying method of the present invention prepares the method for lapatinib tosylate solid dispersion, comprises the following steps: (1) the carrier PVPS630 is polymer material, carries out drug The loading ratio is used to prepare the physical mixture; the drug loading ratio is 1:3. (2) Take the physical mixture (including Lapatinib ditosylate), add it to 30mL acetonitrile solution, and ultrasonically dissolve it completely. The solution is quickly frozen in liquid nitrogen, dried in a freeze dryer for 24 hours, taken out and placed in P 2 o 5 Put in a desiccator for 24 hours, pass through a 100-mesh sieve for subsequent use, and prepare a solid dispersion of lapatinib tosylate. The weight-to-volume ratio of the physical mixture to the acetonitrile solution is 500 mg:100 mL. The volume ratio of acetonitrile and water in the acetonitrile solution is 2:1. The temperature in the freeze dryer is -40...
Embodiment 3
[0034] The difference between embodiment 3 and embodiment 1 is: a kind of freeze-drying method of the present invention prepares the method for lapatinib tosylate solid dispersion, comprises the following steps: (1) the carrier PVPS630 is polymer material, carries out drug The loading ratio is used to prepare a physical mixture; the drug loading ratio is 1:2. (2) Take the physical mixture (including Lapatinib ditosylate), add it to 30mL acetonitrile solution, and ultrasonically dissolve it completely. The solution is quickly frozen in liquid nitrogen, dried in a lyophilizer for 36 hours, taken out and placed in P 2 o 5Put in a desiccator for 48 hours, pass through an 80-mesh sieve for subsequent use, and obtain a solid dispersion of lapatinib tosylate. The weight-to-volume ratio of the physical mixture to the acetonitrile solution is 500mg: 60mL. The volume ratio of acetonitrile and water in the acetonitrile solution is 2:1. The temperature in the freeze dryer is -40°C, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com