Preparation method of anti-tumor indole compound, indole compound and application
A compound and indole technology, applied in the field of chemical synthesis, can solve the problems of difficult preparation, high toxicity and side effects, and low drug efficacy, and achieve the effects of environmental friendliness, low toxicity and side effects, and simple and practical operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The embodiment of the present invention provides a preparation method of an anti-tumor indole compound, comprising the following preparation steps:
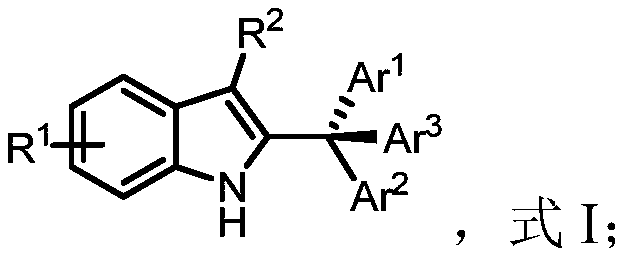
[0038] Obtain the compound of formula II and the compound of formula III, dissolve the compound of formula II and the compound of formula III in an organic solvent, carry out the synthesis reaction under the condition that the temperature is 0°C to 40°C, and the catalyst is chiral phosphoric acid, to obtain the compound of formula I anti-tumor indole compounds;
[0039] Ar 3 H, formula III;
[0040] Among them, R 1 and R 2 Any one independently selected from alkyl, aryl, heteroaryl, substituted aryl and substituted heteroaryl, Ar 1 、Ar 2 and Ar 3 are independently selected from any one of aryl, heteroaryl, substituted aryl and substituted heteroaryl, and Ar 1 、Ar 2 and Ar 3 different from each other.
[0041] The preparation method of the anti-tumor indole compound provided by the embodiment of the present in...
Embodiment 1
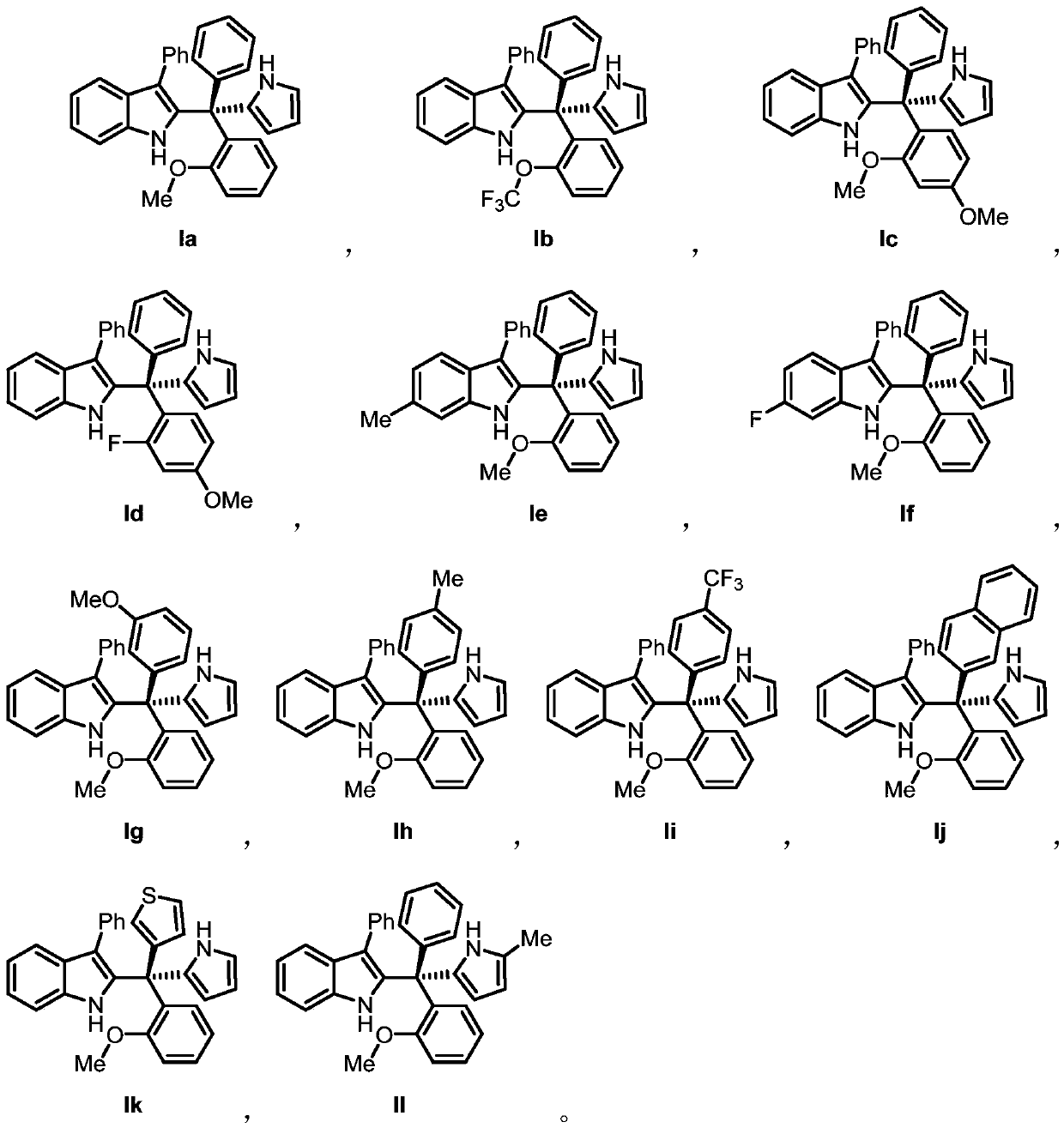
[0070] An indole compound Ia, Including the following preparation steps:
[0071] At room temperature, the R 1 for hydrogen, R 2 is phenyl, Ar 2 is 2-methoxyphenyl, Ar 3 Triarylmethanol (81.3mg, 0.2mmol) and pyrrole (26.8mg, 0.4mmol), which are phenyl groups, were dissolved in dichloromethane (1.6mL), and dichloromethane (14.9mg, 0.015mmol) of chiral phosphoric acid (14.9mg, 0.015mmol) was slowly added dropwise. Methane (0.4 mL) solution was stirred at room temperature for 24 hours, and the reaction solution was directly separated by silica gel column chromatography to obtain Compound Ia, 87.0 mg of white foamy solid, with a calculated yield of 96%.
[0072] In order to further verify that the compound obtained is indeed the target product to be prepared in this embodiment, the obtained product is qualitatively analyzed by measuring the specific rotation, high performance liquid chromatography analysis, ee value, and nuclear magnetic resonance. The analysis of the test is...
Embodiment 2
[0082] A kind of indole compound Ib, Including the following preparation steps:
[0083] At room temperature, the R 1 for hydrogen, R 2 is phenyl, Ar 2 For 2-trifluoromethoxyphenyl, Ar 3 Triarylmethanol (91.9mg, 0.2mmol) and pyrrole (26.8mg, 0.4mmol), which are phenyl groups, were dissolved in dichloromethane (1.6mL), and dichloromethane (14.9mg, 0.015mmol) of chiral phosphoric acid (14.9mg, 0.015mmol) was slowly added dropwise. Methane (0.4 mL) solution was stirred at room temperature for 24 hours, and the reaction solution was directly separated by silica gel column chromatography to obtain compound Ib, 86.5 mg of white foamy solid, and the calculated yield was 85%.
[0084] In order to further verify that the compound obtained is indeed the target product to be prepared in this embodiment, the obtained product is qualitatively analyzed by measuring the specific rotation, high performance liquid chromatography analysis, ee value, and nuclear magnetic resonance. The anal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com