Method for improving recovery rate of epoxidation catalyst
A technology of catalyst and recovery rate, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., to improve recovery rate, improve safety, and reduce the risk of thermal decomposition Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
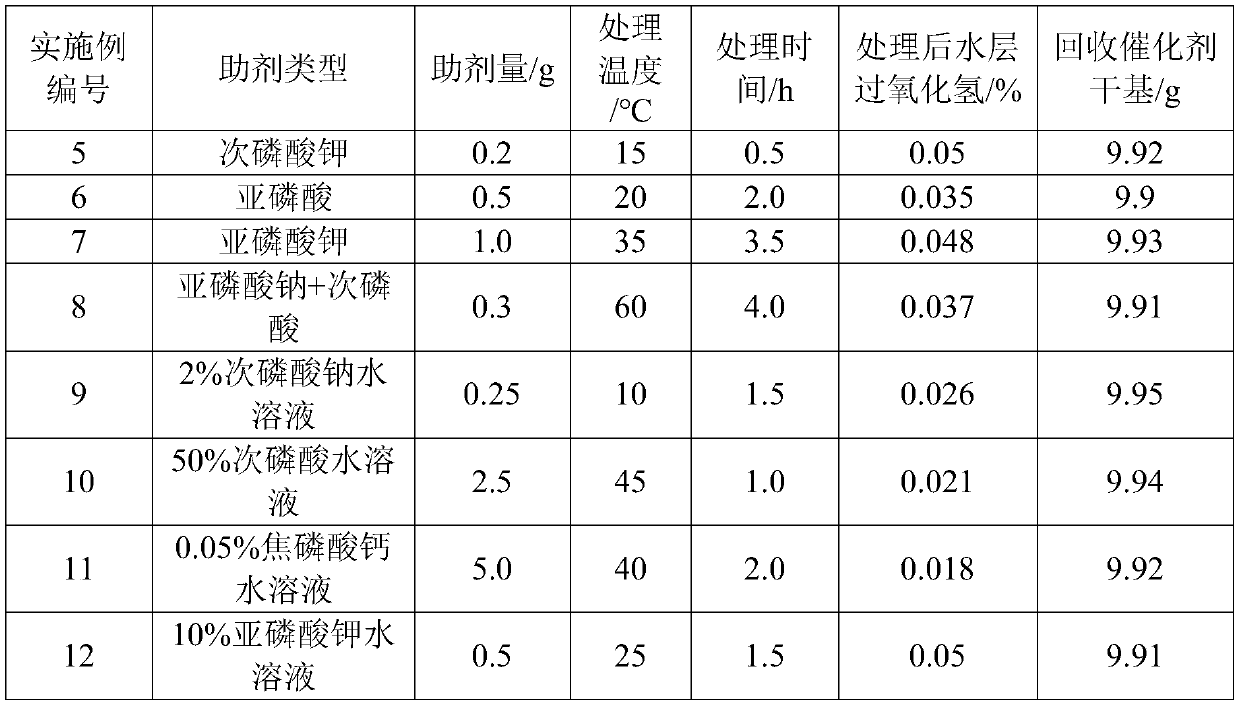
[0013] In a 1L four-necked glass flask, there is 200g of epoxidation reaction solution (10g of catalyst on a dry basis for reaction), and the hydrogen peroxide content of the aqueous layer is 0.6%. Add 0.05g of sodium hypophosphite thereinto, heat up to 45°C, stir and keep warm for 1h, measure The content of hydrogen peroxide in the aqueous phase was 0.03%, and 9.93 g of the catalyst was obtained on a dry basis after separation.
Embodiment 2
[0015] In a 1L four-necked glass flask, there is 300g of epoxidation reaction solution (12g of catalyst on a dry basis for reaction), and the hydrogen peroxide content in the aqueous layer is 0.9%. Add 0.4g of hypophosphorous acid thereinto, heat up to 35°C, stir and keep warm for 1.5h, measure The content of hydrogen peroxide in the aqueous phase was 0.05%, and 11.9 g of the catalyst was obtained on a dry basis after separation.
Embodiment 3
[0017] In the 1L four-necked glass flask, 400g of epoxidation reaction solution (11g of catalyst dry basis) is arranged, and the hydrogen peroxide content of the aqueous layer is 1.1%. Add 2.0g mass concentration 0.5% sodium pyrophosphate aqueous solution and 1.0g mass concentration 2 % pyrophosphoric acid, the temperature was raised to 40°C, stirred and kept for 2 hours, the hydrogen peroxide content in the water phase was measured to be 0.05%, and 10.92g of the catalyst was obtained on a dry basis after separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com