Dammarane triterpenoid glycoside compound as well as preparation method and application thereof
A kind of technology of dammarane triterpene sugar and compound, applied in the field of dammarane triterpene glycoside compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
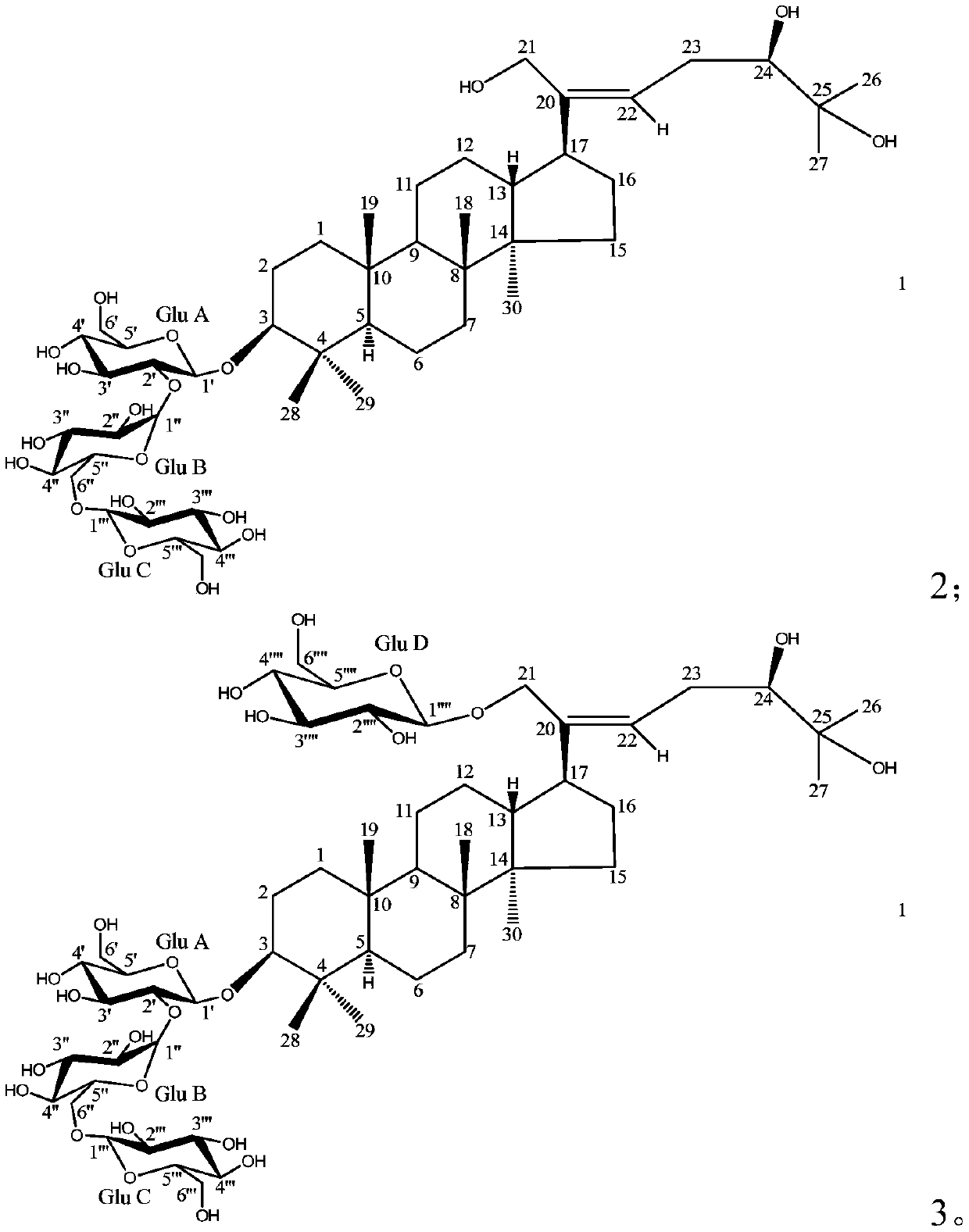
[0039] Embodiment 1: the preparation of compound 1,2,3
[0040] 1) Get the extract
[0041] Weigh 1Kg of dry branches and leaves of Mycetia sinensis (Hemsl.) Craib (Guangxi sweet tea), crush them, add 8 times the weight of 95% (volume) ethanol, heat and reflux for 60 minutes to extract, filter, and collect the filtrate. Add 8 times the weight of 95% (volume) ethanol to the filter residue again, heat and reflux for 60 minutes to extract, and filter. The filtrates were combined, and the filtrate was concentrated to 2 L at 60° C. with a rotary evaporator under reduced pressure. Add 2 L of methanol to the concentrated solution, extract twice with petroleum ether, 2 L each time, collect the lower phase, recover the solvent from the petroleum ether phase and discard it, and rotary evaporate the lower phase to 2 L at 60°C to obtain the extract A001.
[0042] 2) Preliminary purification of the extract
[0043] Take the extract A001 and put it on the X-5 macroporous adsorption resin...
Embodiment 2
[0053] Embodiment 2: Structural characterization of compound 1
[0054] The white amorphous powder A003 of embodiment 1 gained is characterized:
[0055] Mycetioside II: white amorphous powder; UV(MeOH) λmax 190nm (terminal absorption); 1 H(500MHz) and 13 C (125MHz) NMR data are shown in Table 1; electrospray high resolution mass spectrometry (negative ion mode) HRESIMS m / z 799.4811[M-H]-, molecular formula C 42 h 72 o 14 , calculated value 799.4844, molecular weight 801.01, degree of unsaturation 7.
[0056] Table 1: The resulting white powder A003 1 H NMR and 13 C NMR data
[0057]
[0058]
[0059]
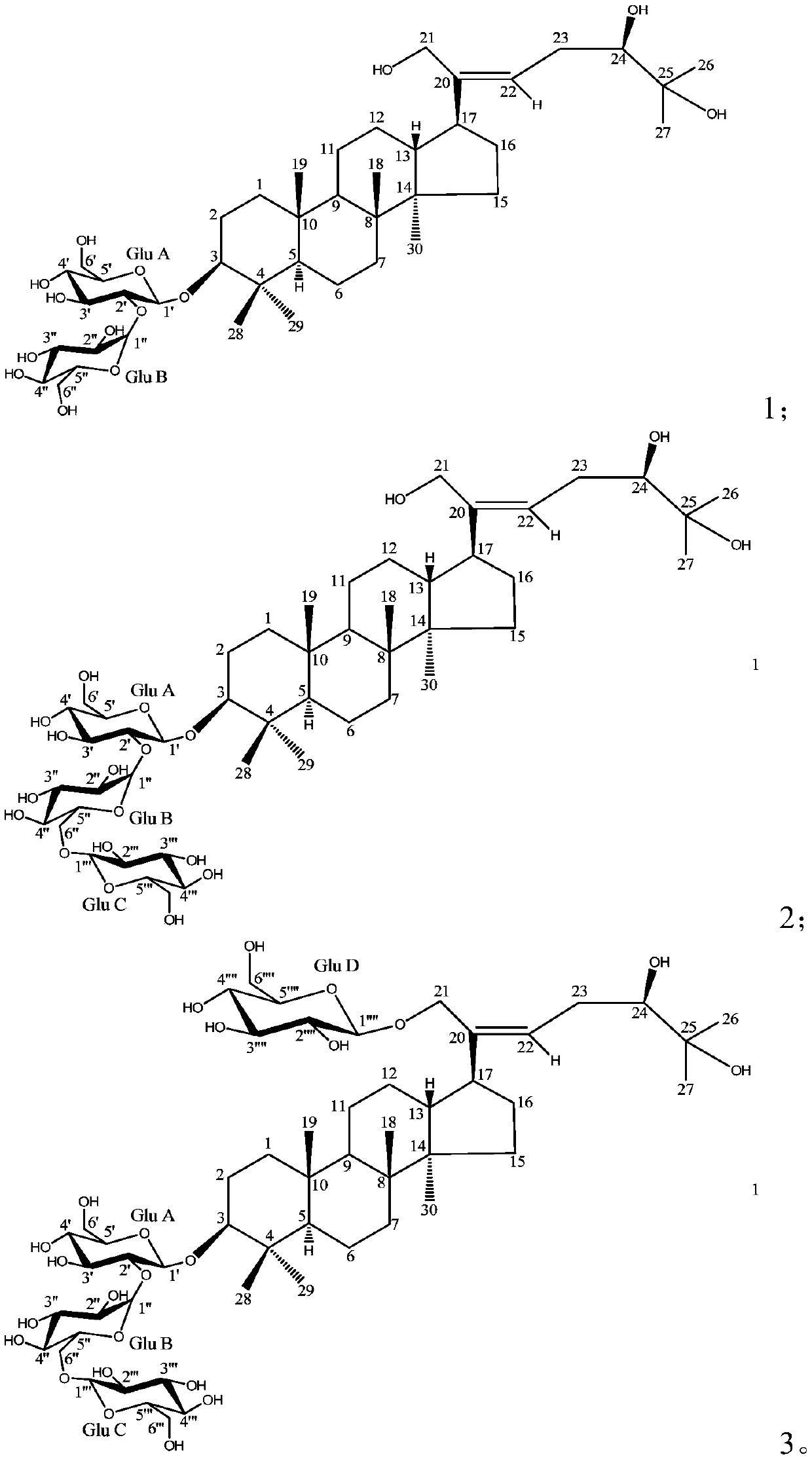
[0060] Therefore, it can be determined that the white powder obtained in Example 1 is compound 1, that is, dammatine II (hereinafter Mycetioside II), and its structure is shown in the following formula 1, and the structure is confirmed by NMR Selective-TOCSY, HSQC, and HMBC.
[0061]
Embodiment 3
[0062] Embodiment 3: Structural characterization of compound 2
[0063] The light yellow amorphous powder A007 obtained in Example 1 is characterized:
[0064] Mycetioside III: light yellow amorphous powder; UV(MeOH) λmax 190nm (terminal absorption); 1 H(500MHz) and 13 C (125MHz) NMR data are shown in Table 2; electrospray high resolution mass spectrometry (negative ion mode) HRESIMS m / z961.5407[M-H]-, molecular formula C 48 h 82 o 19 , calculated value 961.5450, molecular weight 963.15, degree of unsaturation 8.
[0065] Table 2: The resulting pale yellow powder A007 1 H NMR and 13 C NMR data
[0066]
[0067]
[0068]
[0069] Note: The solvent is deuterated methanol, the hydrogen spectrum is 500MHz, and the carbon spectrum is 125MHz.
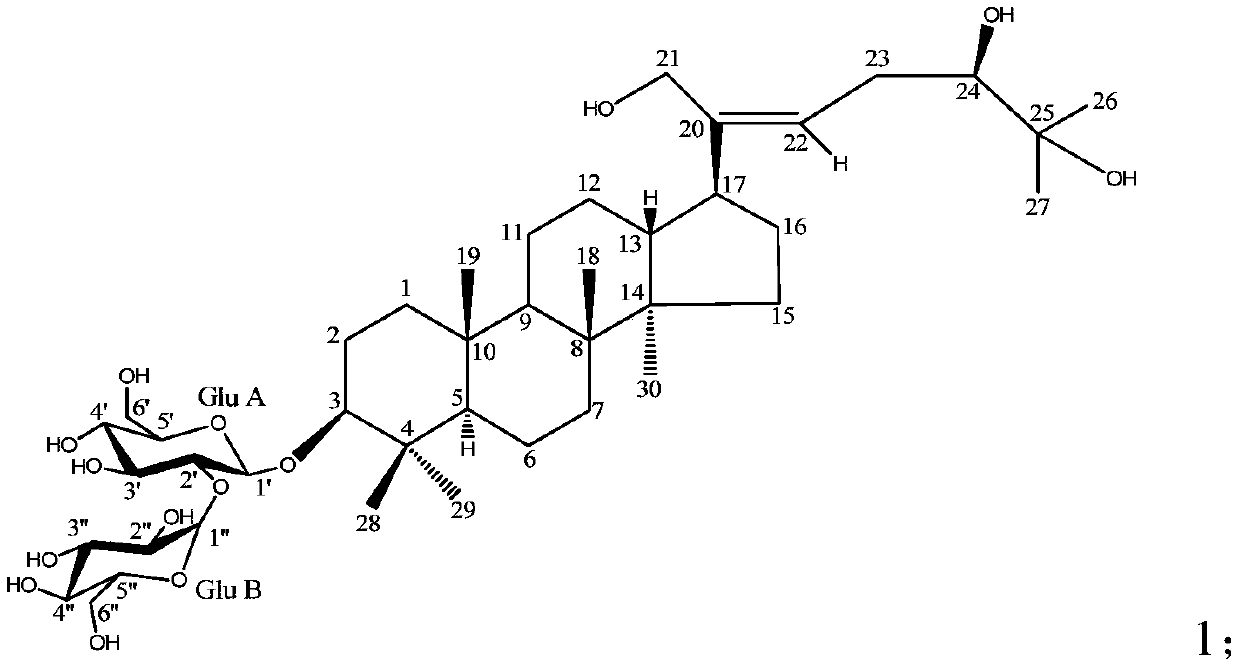
[0070] Therefore, it can be determined that the yellow amorphous powder in Example 1 is compound 2, i.e. dammatine tea glycoside III (Mycetioside III), its structure is as shown in the following formula 2, and this structure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com