A method for producing inositol by complete phosphorylation of cellulose
A technology for inositol and polyphosphoric acid, applied in the field of enzymatic catalysis preparation of inositol, can solve problems such as inability to meet industrialization requirements, increase in inositol production cost, difficulty in separation and purification of inositol, and achieve cheap synthesis and production with low production cost , a rich source of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Production of inositol from cellulose catalyzed by cellopolysaccharide phosphorylase
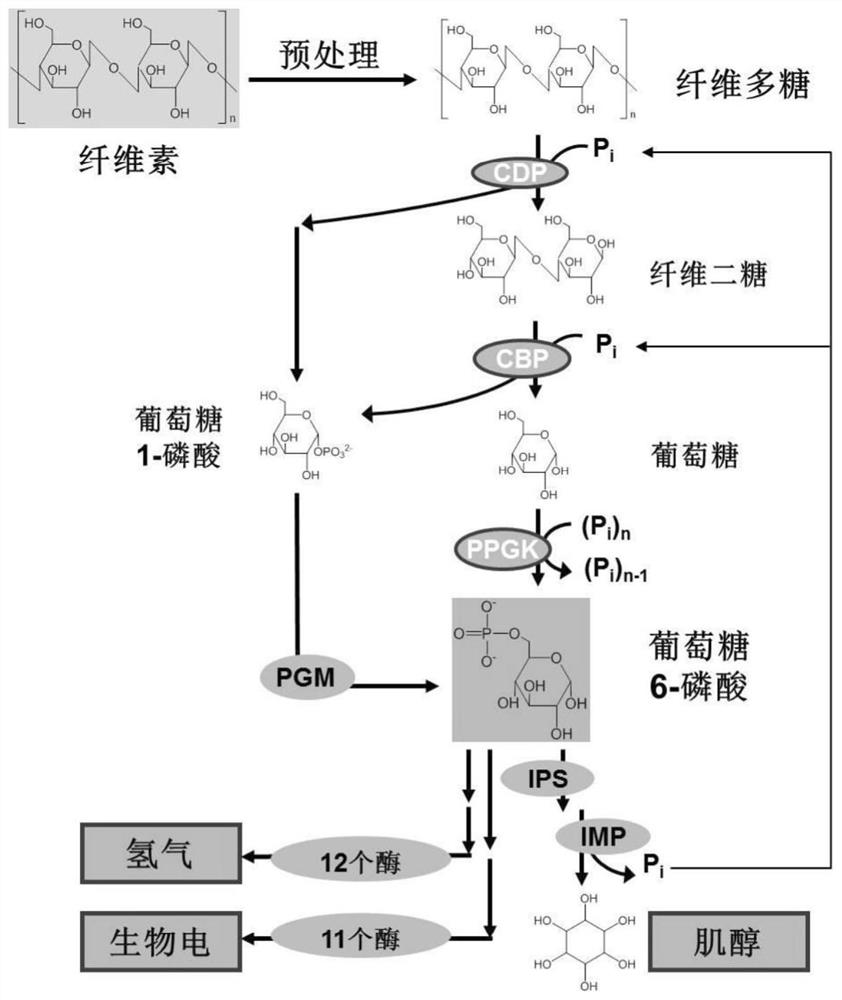
[0058] The catalytic pathways for the complete phosphorylation of cellulose into inositol or the production of hydrogen and electricity through an in vitro multi-enzyme catalytic system are shown in figure 1 . The key enzymes involved in catalyzing the inositol-producing pathway of cellopolysaccharide include: (1) cellopolysaccharide phosphorylase (CDP, EC2.4.1.49), which is used to release glucose 1-phosphate from cellopolysaccharide until cellobiose; ( 2) Cellobiose phosphorylase (CBP, EC 2.4.1.20), used to phosphorylate cellobiose into glucose 1-phosphate and glucose; (3) Polyphosphoglucokinase (PPGK, EC 2.7.1.63) used to Phosphorylation of glucose to glucose 6-phosphate; (4) phosphoglucomutase (PGM, EC 5.4.2.2), for the isomerization of glucose 1-phosphate to glucose 6-phosphate; (5) inositol-1 - phosphate synthase (IPS, EC 5.5.1.4), for the conversion of glucose 6-pho...
Embodiment 2
[0065] Example 2 Utilizing cellopolysaccharide phosphorylase and cellobiose phosphorylase to catalyze the production of inositol from cellulose
[0066] Cellobiose phosphorylase cannot completely phosphorylate cellopolysaccharide to generate glucose 1-phosphate, and adding cellobiose phosphorylase to the reaction system can increase the substrate conversion rate.
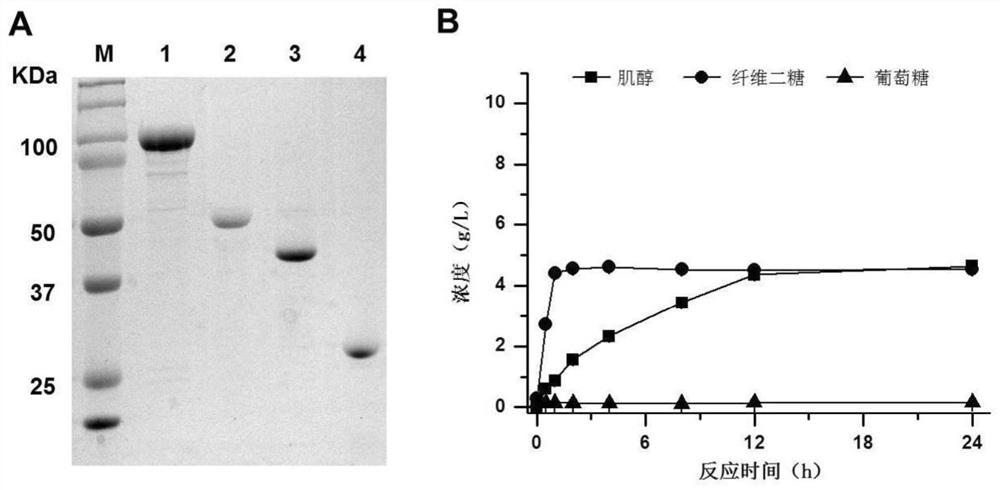
[0067] In this example, cellobiose phosphorylase is derived from Clostridium thermocellum (Clostridium thermocellum), and its gene number is Cthe_0275; the gene is obtained by PCR, and cloned into pET20b vector by Simple Cloning method to obtain corresponding expression Vector pET20b-ctcbp. Then, this plasmid was transformed into Escherichia coli expression strain BL21 (DE3), and protein expression and purification were carried out. The results of protein purification were as follows: image 3 As shown in A. The basic information of the cellobiose phosphorylase used in this example is shown in Table 1.
[0068] A...
Embodiment 3
[0070] Example 3 In vitro multi-enzyme system complete phosphorylation of cellulose to produce inositol
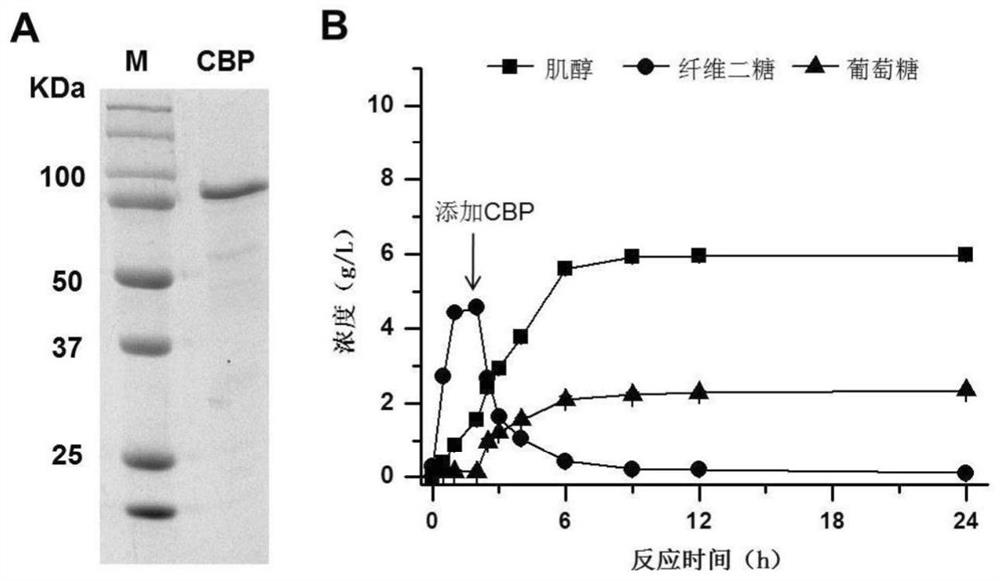
[0071] In order to further increase the conversion rate of inositol, polyphosphoglucokinase (PPGK, EC2.7.1.63) and sodium polyphosphate were added to the reaction system to phosphorylate glucose to generate glucose 6-phosphate, which was then converted into inositol.
[0072] In this example, polyphosphoglucokinase is derived from Thermobifida fusca, and its gene number is Tfu_1811; the gene is obtained by PCR, and cloned into pET20b vector by Simple Cloning method to obtain corresponding expression Vector pET20b-tfuppgk. The polyphosphate glucokinase used in this example is the mutant 4-1 (Zhou W, Huang R, Zhu Z, Zhang Y.-H.P.J., Coevolution of both thermostability and activity of polyphosphate glucokinase from Thermobifida fusca) after thermostability modification YX.Appl.Environ.Microbiol.2018.doi:10.1128 / AEM.01224-18), protein expression and purification results are a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com