Synthesis method of 1-hydroxycyclopropane carboxylic acid and 1-hydroxycyclopropane carboxylate
A technology of hydroxycyclopropane carboxylic acid and synthesis method, which is applied in the direction of carboxylate preparation, carboxylate/lactone preparation, separation/purification of carboxylic acid compounds, etc., and can solve large pollution, low yield and harsh reaction conditions and other problems, to achieve the effects of mild and controllable reaction conditions, simple post-processing, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
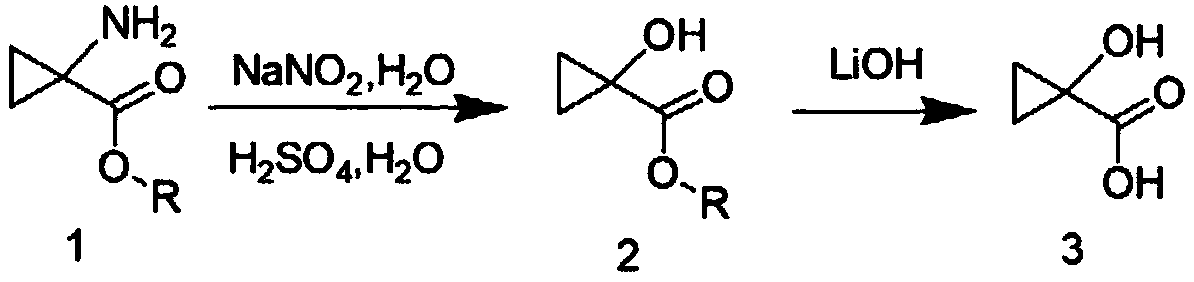
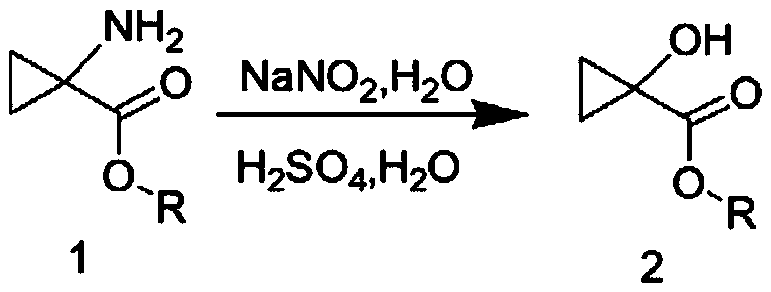
[0035] (1) Synthesis of Compound 2
[0036]
[0037] Dissolve compound 1 (3.41g, 29.6mmol, 1.0eq) in 40ml of sulfuric acid aqueous solution A (prepared by adding 1.62mL of 98% concentrated sulfuric acid to water, the molar equivalent of sulfuric acid is 1.0eq), and cool down to 0-5°C in an ice bath , Add 10ml of sodium nitrite (2.25g, 32.6mmol, 1.1eq) aqueous solution to the reaction solution, and stir at room temperature for 1 hour. Then the above reaction solution was added dropwise to refluxed 100ml sulfuric acid aqueous solution B (prepared by adding 1.62mL of 98% concentrated sulfuric acid into water, the molar equivalent of sulfuric acid was 1.0eq). After the dropwise addition was completed, the heating was stopped and cooled to room temperature. After the reaction was detected by TLC, ethyl acetate (3*100mL) was extracted three times, the organic phases were combined, dried with anhydrous magnesium sulfate, filtered, and the organic phase was concentrated to obtain c...
Embodiment 2
[0044] (1) Synthesis of Compound 2
[0045] Compound 1 (1200.0 g, 10.4 mol, 1.0 eq) was dissolved in 12 L of sulfuric acid aqueous solution (prepared by adding 0.57 L of 98% concentrated sulfuric acid into water, the molar equivalent of sulfuric acid was 1.0 eq), cooled to 0-5 °C in an ice bath, and 3.5 L of sodium nitrite (793.5 g, 11.5 mol, 1.1 eq) aqueous solution was added to the reaction liquid, and stirred at room temperature for 1 hour. Then the above reaction solution was added dropwise to 35L of refluxing sulfuric acid aqueous solution B (prepared by adding 0.57L of 98% concentrated sulfuric acid into water, and the molar equivalent of sulfuric acid was 1.0eq). After the addition was completed, stop heating, cool to room temperature, and detect by TLC After the reaction was completed, ethyl acetate (3*12L) was extracted three times, the organic phases were combined, dried with anhydrous magnesium sulfate, filtered, and the organic phase was concentrated to obtain comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com