Allograft tolerance without the need for systemic immune suppression
A technology of allograft and graft, applied in the field of transplantation, can solve problems such as systemic immune suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0407] Example 1: Materials and methods
[0408] Construction of vectors expressing target genes essential for allogeneic tolerance
[0409] The plasmid containing the cDNA sequence of the gene involved in allogeneic tolerance was obtained as follows:
[0410] PD-L1: Mount Sinai Hospital, clone #V102001
[0411] FasL: Mount Sinai Hospital, #75719
[0412] Cd47: Mount Sinai Hospital, #V75535
[0413] Cd200: GE Dharmacon, ID#17470
[0414] H2-M3: Mount Sinai Hospital, clone #8188
[0415] Ccl21: Mount Sinai Hospital, clone #V77120
[0416] Mfge8: Mount Sinai Hospital, clone #V72614
[0417] Spi6: Mount Sinai Hospital, clone #V8907
[0418] The Gateway cloning system (Thermo Fisher) was used to generate expression vectors containing these target genes (GOI) or luciferase. Cd47, Ccl21, Mfge8 and Spi6 cDNA were obtained in the form of containing cDNA flanking attB sites. For H2-M3, Cd200, FasL and PD-L1, design primers to amplify the cDNA sequence and add attB sites ( Figure 15 (a)). After PCR...
Embodiment 2
[0433] Example 2: Generation of masked cells
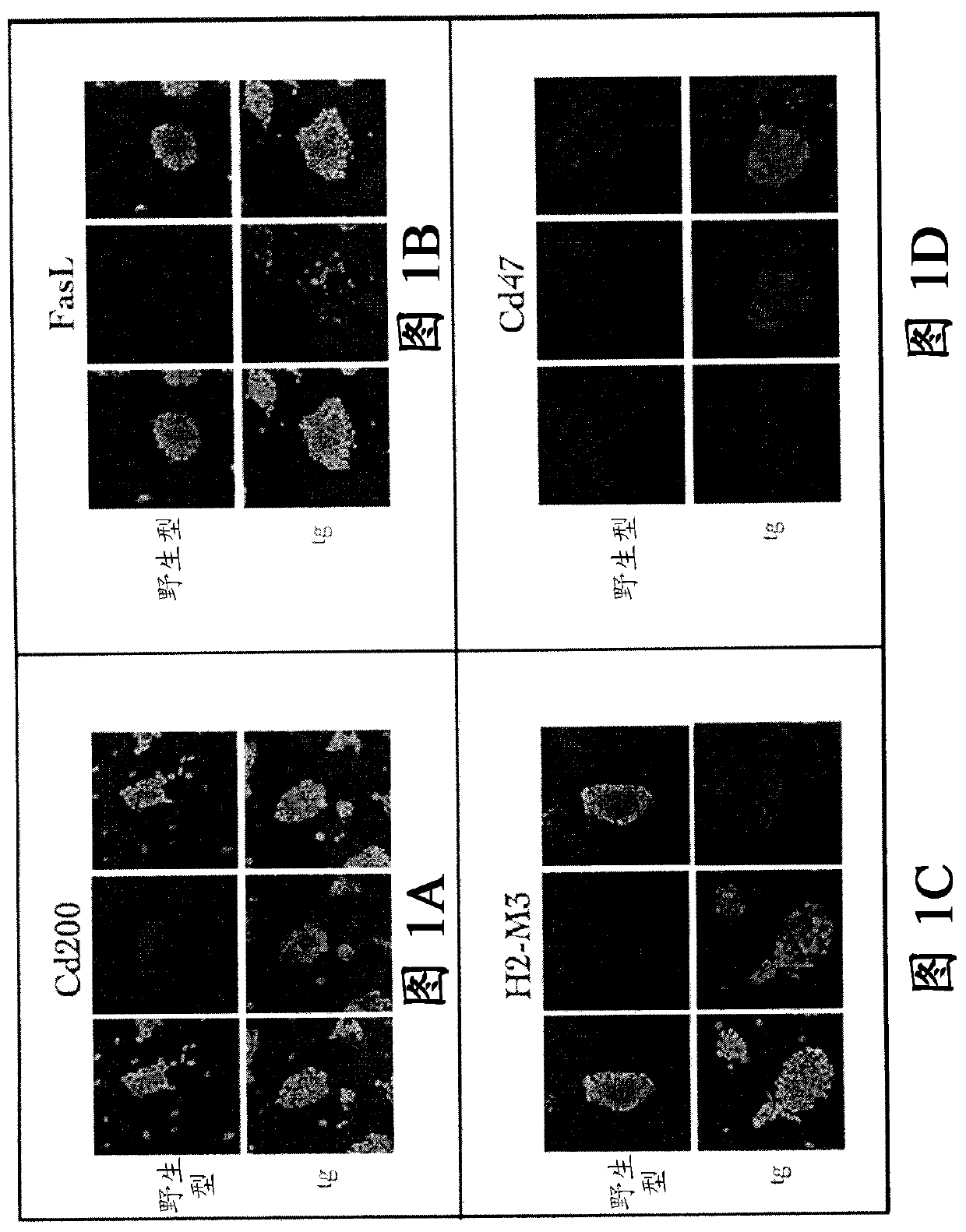
[0434] The transgenes encoding the genes in Table 1 were cloned into an expression vector, and sequence verification was performed by polymerase chain reaction (PCR), restriction enzyme digestion, and sequencing, all using standard methods known in the art.
[0435] A group of constructs (group 1) containing transgenic Cd47, Cd200, FasL and H2-M3 were transfected into mouse embryonic stem cells derived from the inventor's C57BL / 6 mouse ES line (C2). The existence of the transgene was verified by PCR, and the expression of the expressed protein was recorded by immunohistochemistry ( Figure 1A -D). The second group of constructs (group 2) containing the transgenes Ccl21, Mfge8, TGF-β and Spi6 was transfected into FVB / N-derived ES cells (ES line C2).
[0436] A similar method was used to generate masked B16F10 melanoma cells except that the medium used DMEM medium containing 10% fetal bovine serum (FBS).
Embodiment 3
[0437] Example 3: Screening method for inhibiting T cell activation
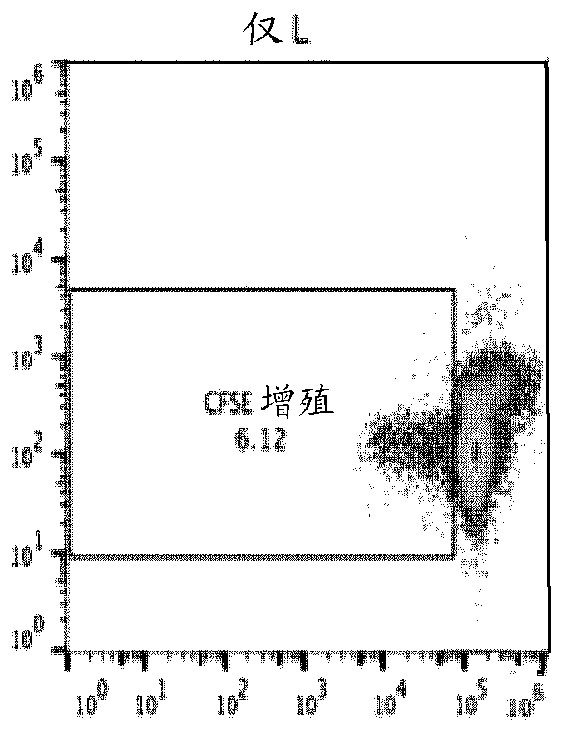
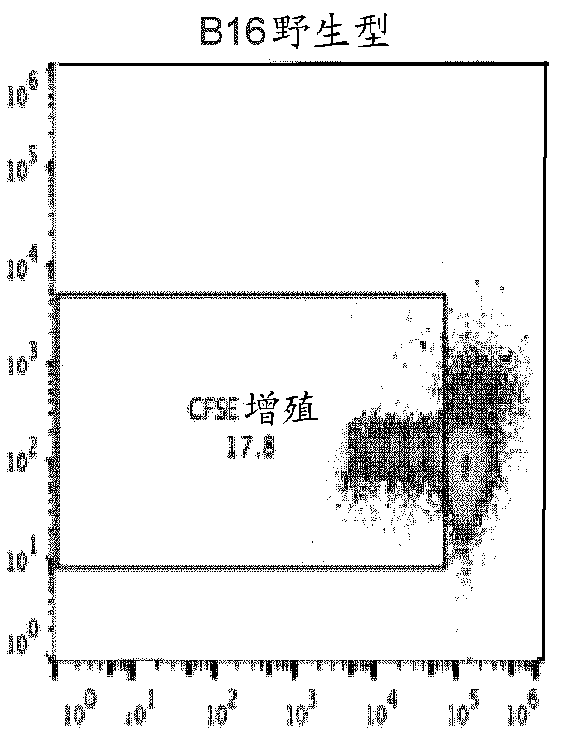
[0438] A modified in vitro mixed lymphocyte response (MLR) assay is used to screen transgenic combinations to produce the most effective inhibition of T cell activation. Cell lines transfected with the masked transgene from Example 1 were used. The donor OT-1 splenocytes were labeled with carboxyfluorescein succinimidyl ester CFSE, and 60,000 cells were added to each well of a 96-well plate. Mix ES or melanoma cells with egg cell expressing cells 10:1. Add 10,000 of these to each well of spleen cells. IL-2 was added as a general activator, and T cell proliferation was measured by flow cytometry after 3 days ( Figure 2A-2E ). The cells were initially gated to include only CD8+ cells, and all conditions were set in quadruplicate.
[0439] The negative control (spleen cells only) produced a baseline 6.12% proliferation rate ( Figure 2A ). Wild-type B16 melanoma (+10% egg cell expression) cells resulted in a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com