Application of sanguinarine in inhibition and removal of multi-drug-resistant providencia rettgeri biofilm
A technology of Providencia rettii organisms and Providencia organisms, which is applied in the direction of antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problem of sanguinarine inhibition and Eliminate problems such as scavenging effect, and achieve the effect of wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be described in further detail below in conjunction with the examples. The examples are only used to explain the content of the present invention, not to limit the present invention.
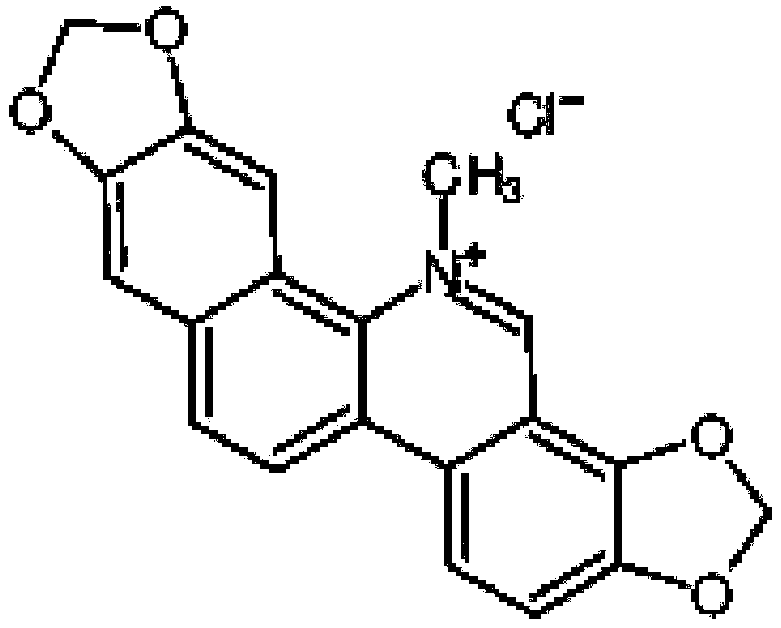
[0020] The English name of sanguinarine is Sanguinarine, and its molecular formula is C 20 h 14 ClNO 4 , the molecular weight is 367.78, the melting point is 281-285°C, and the CAS number is 5578-73-4. The molecular structure is:
[0021]
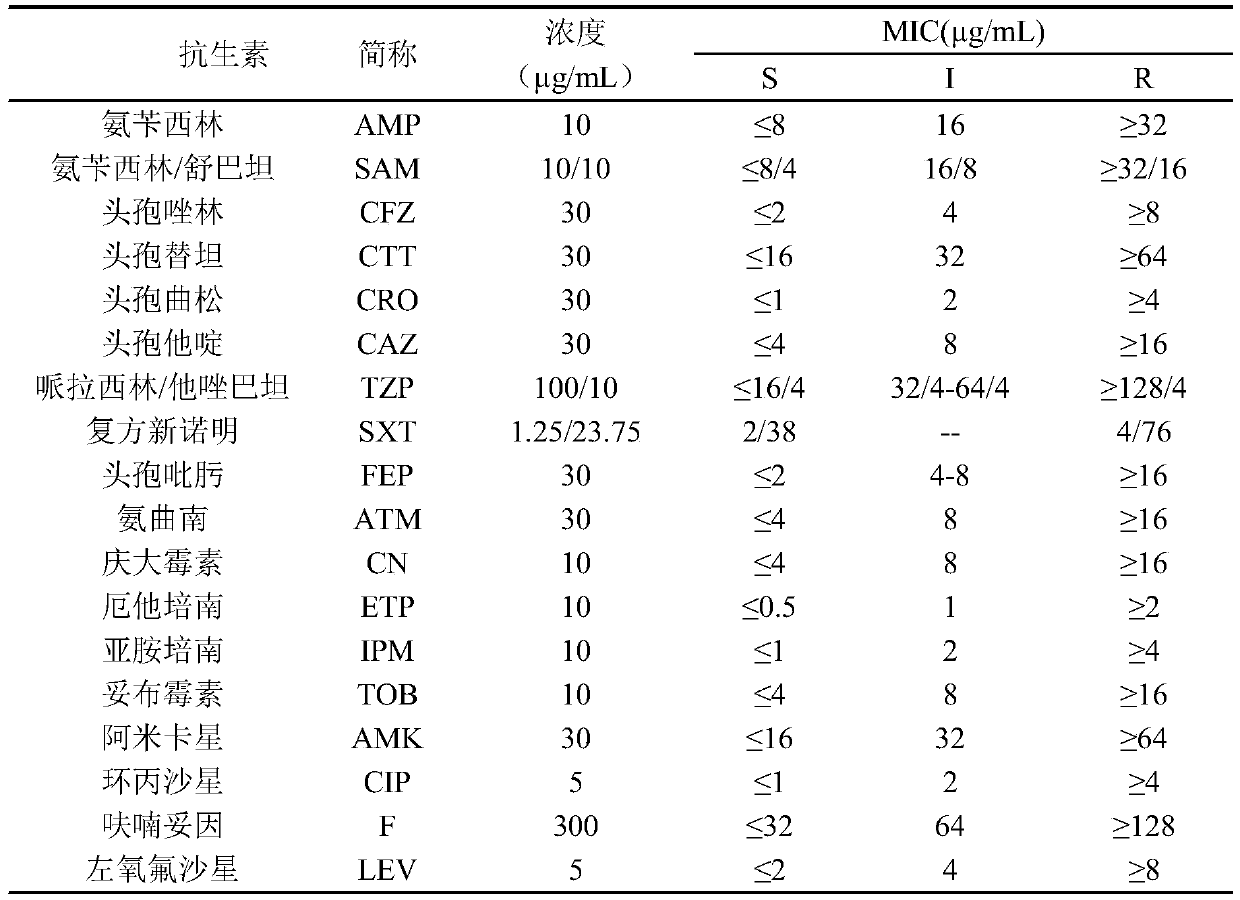
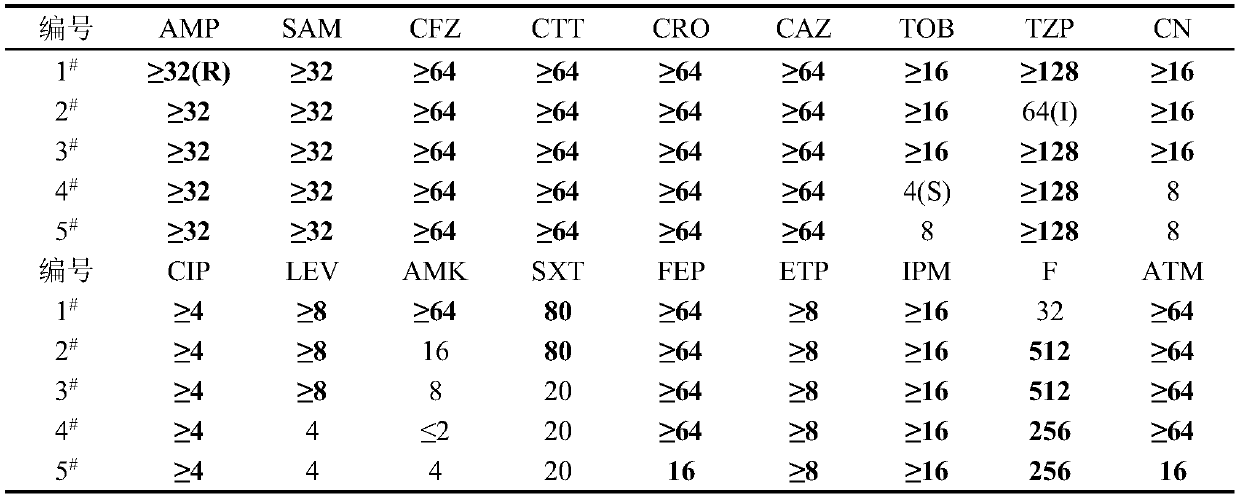
[0022] 1. Drug susceptibility test of Providencia rettgeri
[0023] The present invention takes 5 strains of Providencia rettgeri as starting strains, selects ampicillin, ampicillin / sulbactam, cefazolin, cefotetan, ceftriaxone, ceftazidime, tobramycin, piperacillin Zobactam, ciprofloxacin, levofloxacin, amikacin, cotrimoxazole, cefepime, ertapenem, imipenem, nitrofurantoin, aztreonam, gentamicin and other 18 kinds of commonly used antibiotics for testing.
[0024] Pick and culture the pure colonies for 24 hours and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com