Humanized anti-CD47 monoclonal antibody and application thereof
A monoclonal antibody, humanized technology, applied in the application, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of not completely eliminating NHL and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0137] The preparation method of the humanized anti-CD47 monoclonal antibody provided by the invention comprises:
[0138] Step 1: Antibody humanization, through sequence alignment on NCBI tools, humanization transformation is completed, and the transformation antibody is screened;
[0139] Step 2: Affinity maturation is performed on the screened humanized antibodies by phage display method to prepare humanized anti-CD47 monoclonal antibodies.
[0140] Specifically, the method includes:
[0141] The preparation method of the CD47 humanized antibody is as follows: using the murine antibody 059-1.43.1 prepared in the patent CN106084052A as a template, PCR amplification obtains the antibody heavy chain variable region gene VH and light chain variable region gene VL, Translate it into an amino acid sequence, then compare the amino acid sequence with the human antibody sequences in the NCBI database, and select the 5 human antibody sequences with the highest similarity to the vari...
Embodiment 1
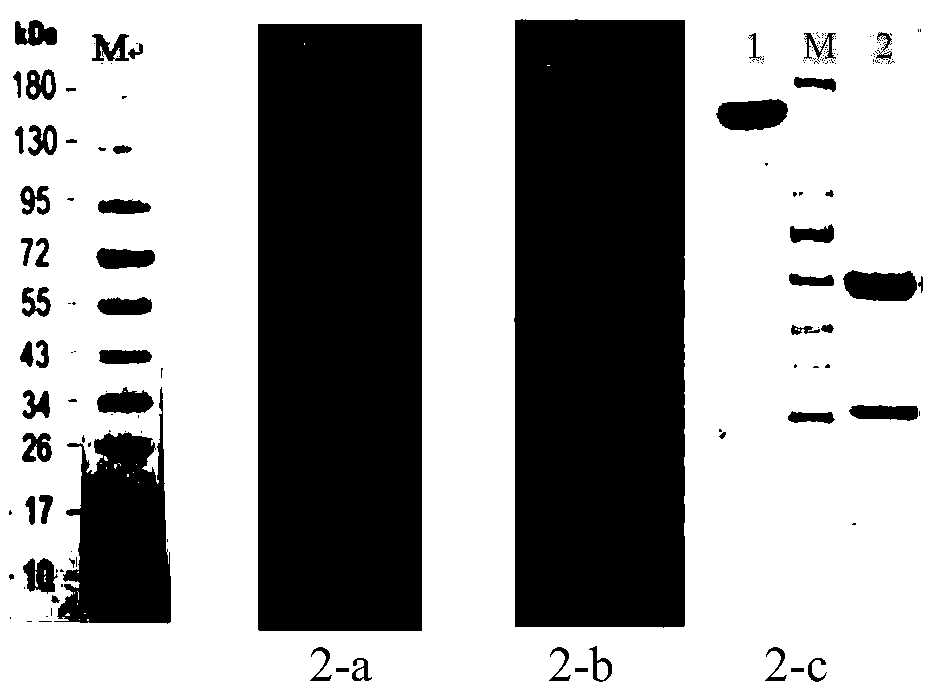
[0143] The preparation of embodiment 1 antigenic protein, chimeric antibody and positive control antibody
[0144] 1. Antigen gene synthesis and expression vector construction
[0145] The amino acid sequence of the extracellular region of the human CD47 protein is fused with the amino acid sequence of the connecting peptide-hIgG1Fc or the connecting peptide-7his, and the amino acid sequence design is as follows:
[0146] The amino acid sequence of human CD47-hIgGFc is as follows (SEQ ID NO:47):
[0147] MWPLVAALLLGSACCGSAQLLFNKTKSVEFTFCNDTVVIPCFVTNMEAQNTTEVYVKWKFKGRDIYTFDGALNKSTVPTDFSSAKIEVSQLLKGDASLKMDKSDAVSHTGNYTCEVTELTREGETIIELKYRVVSWFSPSGGGGSASEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
[0148] The amino acid sequence of human CD47-7His is as follows (SEQ ID NO: 48):
[0149] MWPLVAA...
Embodiment 2
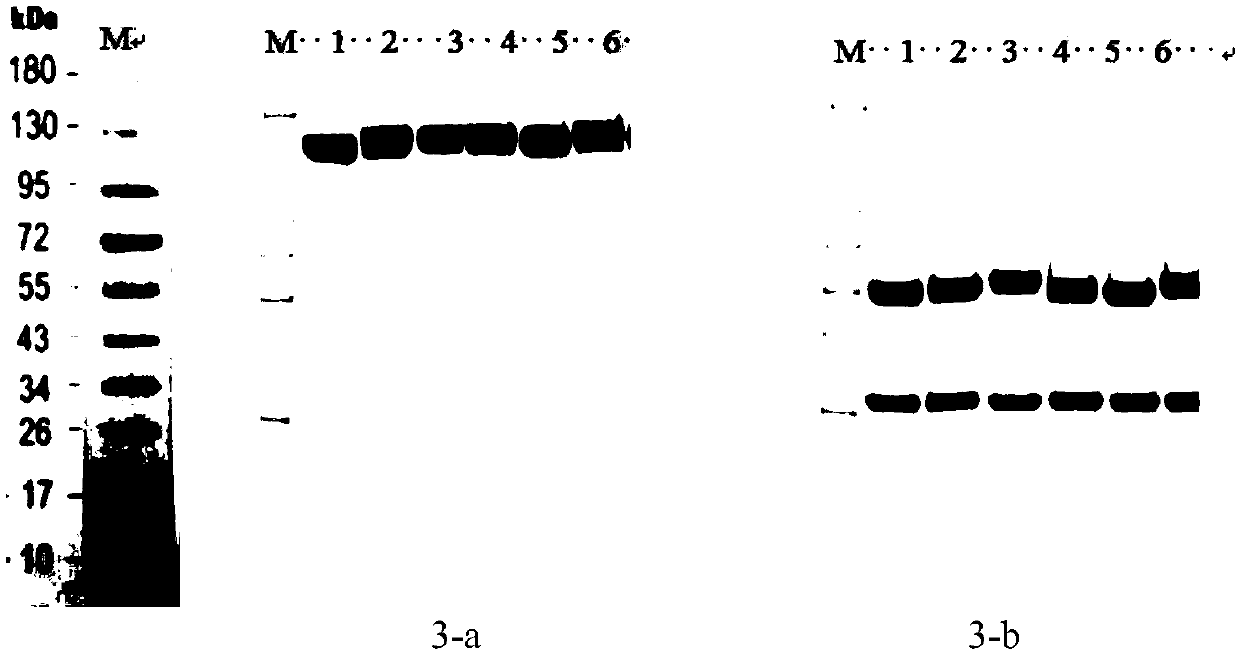
[0174] Embodiment 2 Humanization
[0175] The murine antibody 059-1.43.1 in the patent CN106084052A was selected for humanization. The humanization process mainly includes human template search and transplantation.
[0176] The main target of humanization transformation is the FR sequence in the variable region. Using the amino acids of the murine antibody 059-1.43.1 VH and VL in patent CN106084052A as templates, sequence alignment was performed on the NCBI website, and 5 humanized reference sequences were found, which were used as reference templates for humanized transformation of antibody FR regions Design humanized transformation sequences.
[0177] The specific sequence of the CDR region is shown in Table 1, the specific sequence of Blastp is shown in Table 2, and the sequence of the humanized antibody after transplantation with the retained FR region is shown in Table 3.
[0178] Table 1. The specific sequence of the CDR region of the 059-1.43.1 antibody
[0179]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com