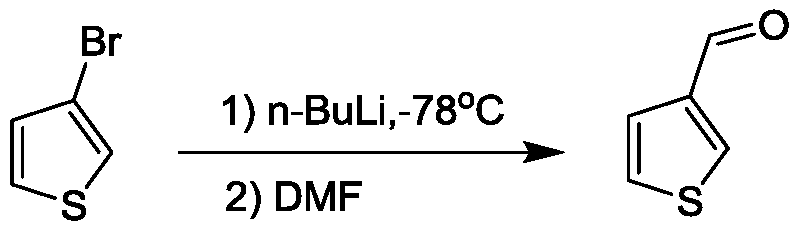
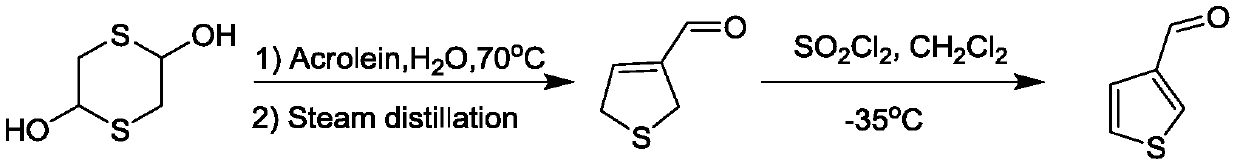
Preparation method of 3-thiophenecarboxaldehyde
A thiophene formaldehyde, methyl thiophene technology, applied in the direction of organic chemistry, can solve the problems of high equipment and temperature requirements, difficult industrial production, high cost, and achieve the effects of avoiding pollution, simple operation, and saving raw material costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
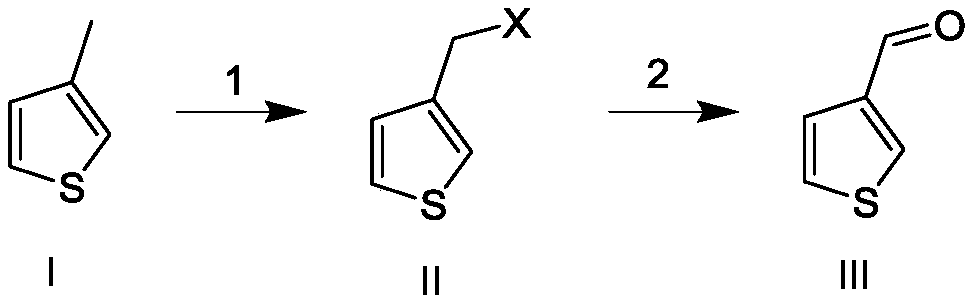
[0035] The preparation method as described herein is carried out according to the following reaction equation:
[0036]
[0037] in,
[0038] 1: Mix 3-methylthiophene and an organic solvent and heat to the first temperature, keep the first temperature constant, add azobisisobutyrocyanide and N-halogenated succinimide respectively, in the After being kept warm for a period of time at the first temperature, the temperature is lowered, and then filtered to obtain a mixed solution of 3-halomethylthiophene;
[0039] 2: Add N-methylmorpholine-N-oxide to the mixed solution of 3-halomethylthiophene obtained above, heat it to the second temperature, then cool down, wash with water, and remove the solvent to obtain the crude product, and further rectify , that is, the pure product of 3-thiophenecarbaldehyde is obtained.
[0040] Specifically as described in the following examples.
Embodiment 1
[0042] Add 35.2g of 3-methylthiophene and 206g of ethyl acetate to the three-necked flask, heat the system to 70°C, then add 11.71g of azobisisobutyrocyanide and 45.23g of N-chlorosuccinyl in batches at this temperature For imine (NCS), after the addition was completed, the temperature was lowered at 70°C for 2 hours, and the precipitated solid was removed by filtration to obtain an ethyl acetate solution of 3-chloromethylthiophene.
[0043]Add 125.3 g of NMO solid (N-methylmorpholine-N-oxide) to the above ethyl acetate solution of 3-chloromethylthiophene, and heat the system to 50°C for 6 hours. Washing with water and precipitation to obtain 23.6 g of 3-thiophenecarbaldehyde with a yield of 58.69%, and further rectification to obtain pure 3-thiophenecarbaldehyde.
Embodiment 2
[0045] Add 37.6g of 3-methylthiophene and 231g of ethyl acetate to the three-necked flask, heat the system to 70°C, then add 12.58g of azobisisobutyrocyanide and 64.76g of N-bromobutylene in batches at this temperature Imide (NBS), after the addition of imide (NBS), the temperature was lowered at 70°C for 2 hours, and the precipitated solid was removed by filtration to obtain an ethyl acetate solution of 3-bromomethylthiophene.
[0046] Add 134.61 g of NMO solid (N-methylmorpholine-N-oxide) to the ethyl acetate solution of the above-mentioned 3-bromomethylthiophene, and heat the system to 50°C for 6 hours. Washing with water and solvent removal gave 26.7 g of 3-thiophenecarbaldehyde with a yield of 62.16%. Further rectification gave pure 3-thiophenecarbaldehyde.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com