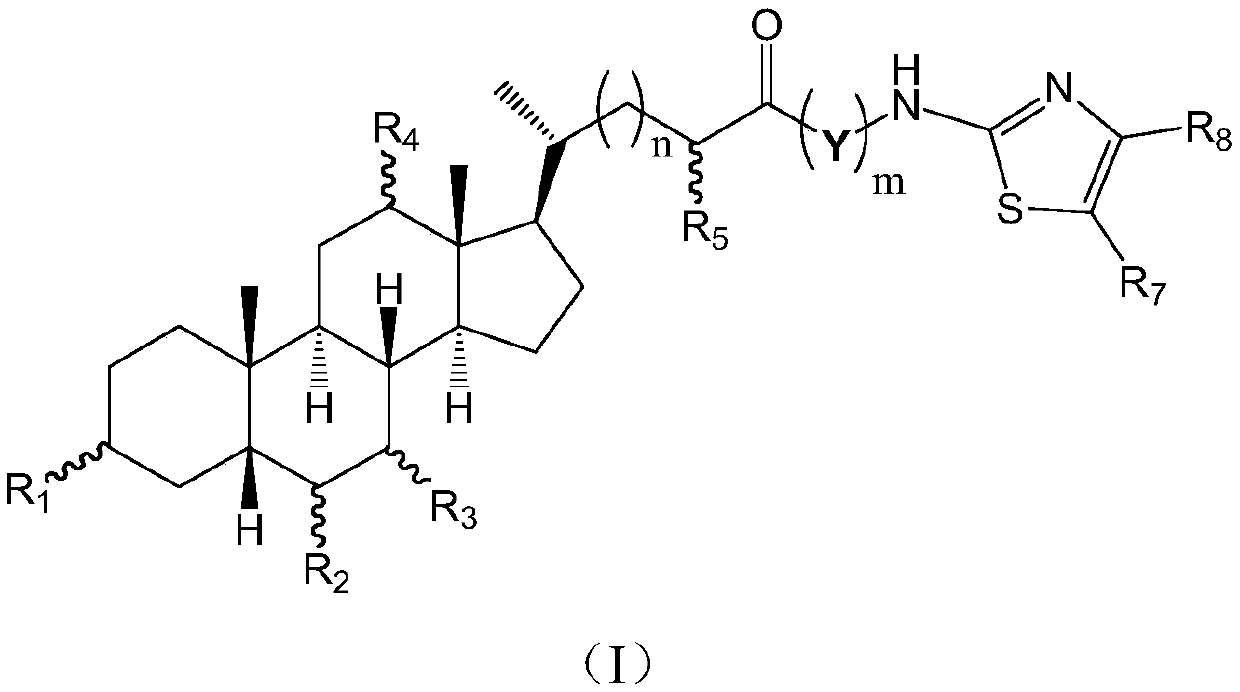
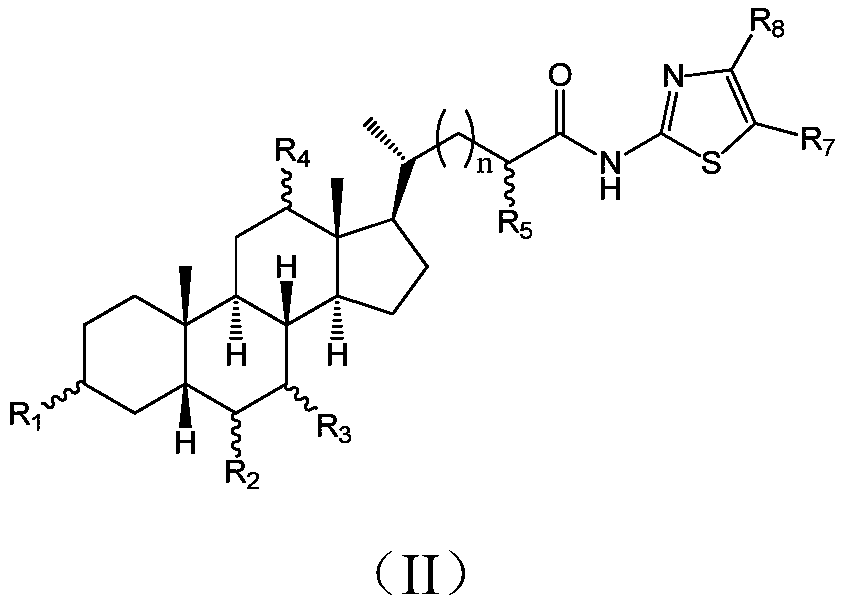
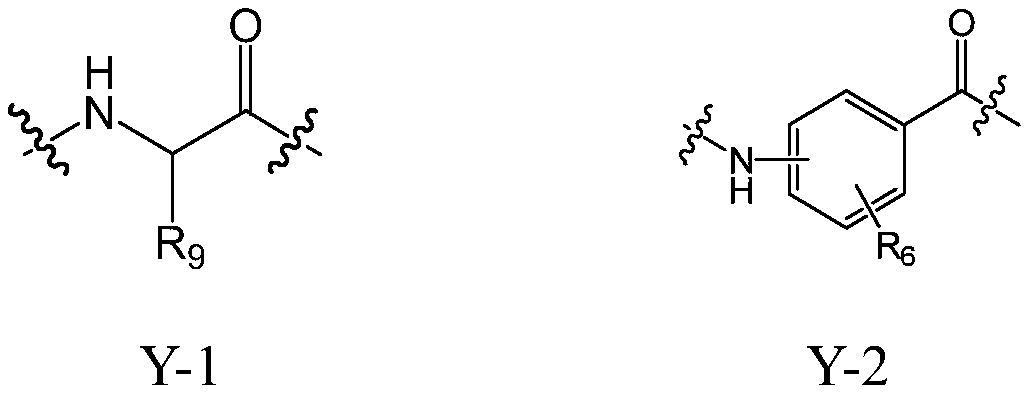Cholic acid derivative with antibacterial activity and pharmaceutical composition thereof
A technology of compounds and solvates, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the synthesis of compound 1-1:
[0076]
[0077] Add 1 g of nor-CDCA, 15 ml of DMF (N,N-dimethylformamide), 0.86 g of TBTU (benzotriazole tetramethyltetrafluoroboric acid), 1 g of DIPEA (N, N-diisopropylethylamine) and 0.35 g of 2-amino-5-nitrothiazole, stirred at room temperature for 5-6 hours, TLC monitored the progress of the reaction until the nor-CDCA reaction was complete, added 50 ml of water and 50 ml of ethyl acetate , after stirring, stand still, separate the organic phase, extract the water phase with ethyl acetate twice and combine, the organic phase is washed successively with 10 ml of 2% dilute hydrochloric acid, 1% potassium carbonate aqueous solution, and saturated brine, dried, and concentrated in vacuo. The residue was subjected to silica gel column chromatography and washed with petroleum ether / ethyl acetate system to obtain 0.8 g of a light yellow solid.
[0078] 1 HNMR (400MHz, CDCl 3 )δppm:8.33(s,1H),3.90(s,1H),3.61-3.46(m,2H), ...
Embodiment 2
[0080] Embodiment 2: the synthesis of compound 1-2:
[0081]
[0082] The raw materials were changed and synthesized by the same method as in Example 1.
[0083] 1 HNMR (400MHz, CD 3 OD) δppm: 8.40(s, 1H), 4.01-3.95(m, 1H), 3.82(q, J=3.0Hz, 1H), 3.46-3.33(m, 1H), 2.61(ddd, J=14.6, 9.9 ,4.7Hz,1H),2.48(ddd, J=15.1,8.8,6.4Hz,1H),2.38-2.23(m,2H),2.09-1.74(m,8H),1.73-1.30(m,7H), 1.26(t,J=7.1Hz,1H),1.22-0.95(m,2H),1.08(d,J=5.8Hz,3H),0.94(s,3H), 0.74(s,3H);
[0084] ESI-MS m / z:534.01[M-1] - .
Embodiment 3
[0085] Embodiment 3: the synthesis of compound 1-3:
[0086]
[0087] The raw materials were changed and synthesized by the same method as in Example 1.
[0088] 1 HNMR (400MHz, CD 3 OD) δppm: 8.39(s, 1H), 3.82(q, J=2.8Hz, 1H), 3.46-3.34(m, 1H), 2.60(ddd, J=15.0, 9.8, 5.1Hz, 1H), 2.47( ddd,J=15.3,9.3,6.6Hz,1H),2.29(d,J=11.5Hz,1H),2.08-1.94(m,3H),1.92-1.81(m,3H), 1.73-1.37(m, 8H), 1.37-1.09(m, 4H), 1.02(d, J=6.4Hz, 3H), 1.05-1.96(m, 1H), 0.90(s, 3H), 0.72(s, 3H);
[0089] ESI-MS m / z:518.01[M-1] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com