Cefoperazone sodium and sulbactam sodium powder for injection and production method of cefoperazone sodium and sulbactam sodium powder for injection
A technology for sofoperazone sodium and sulbactam sodium powder and cefoperazone sodium is applied in the field of cefoperazone sodium and sulbactam sodium powder injection for injection and its preparation, and can solve the problem of reducing the content of active pharmaceutical ingredients, unstable beta-lactam ring, and storage conditions. Strict requirements, etc., to achieve the effect of reducing dosage, good antibacterial effect, reducing degradation and polymerization reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
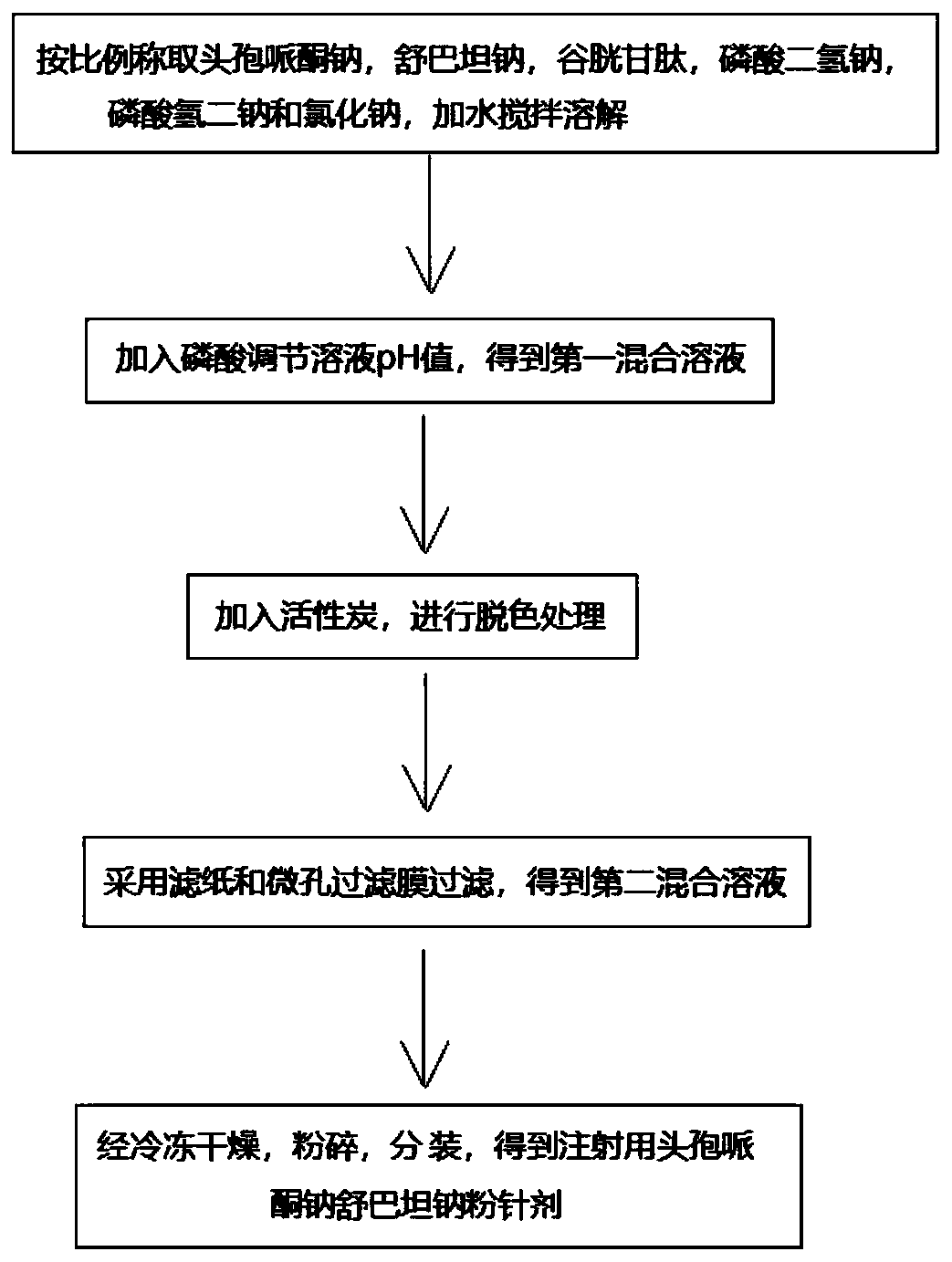
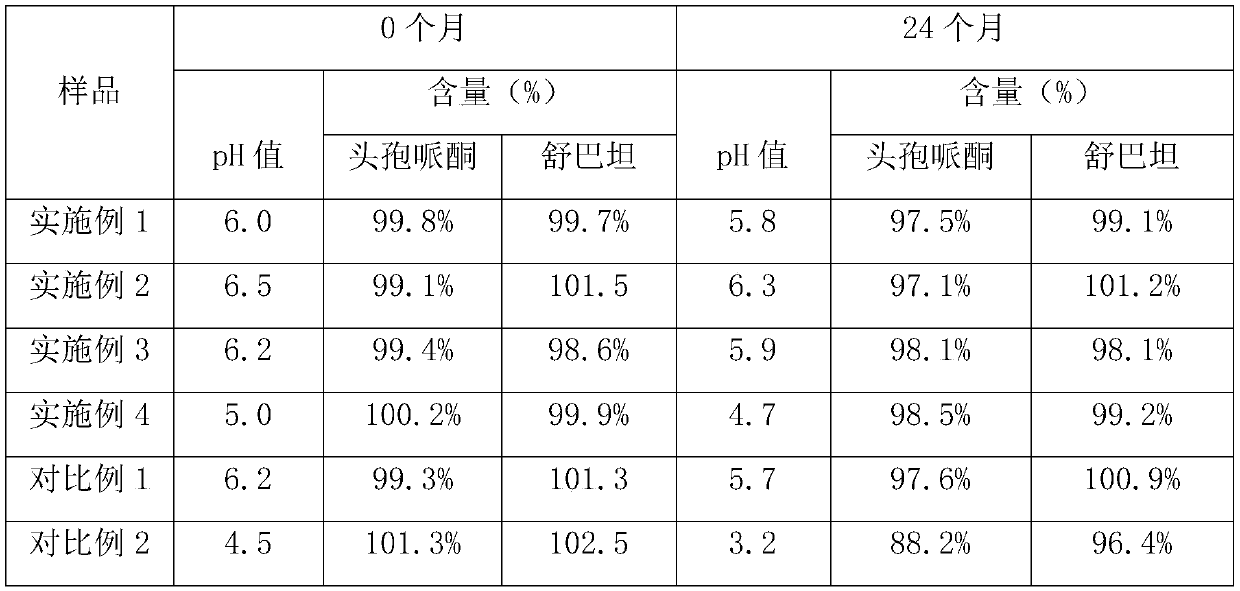
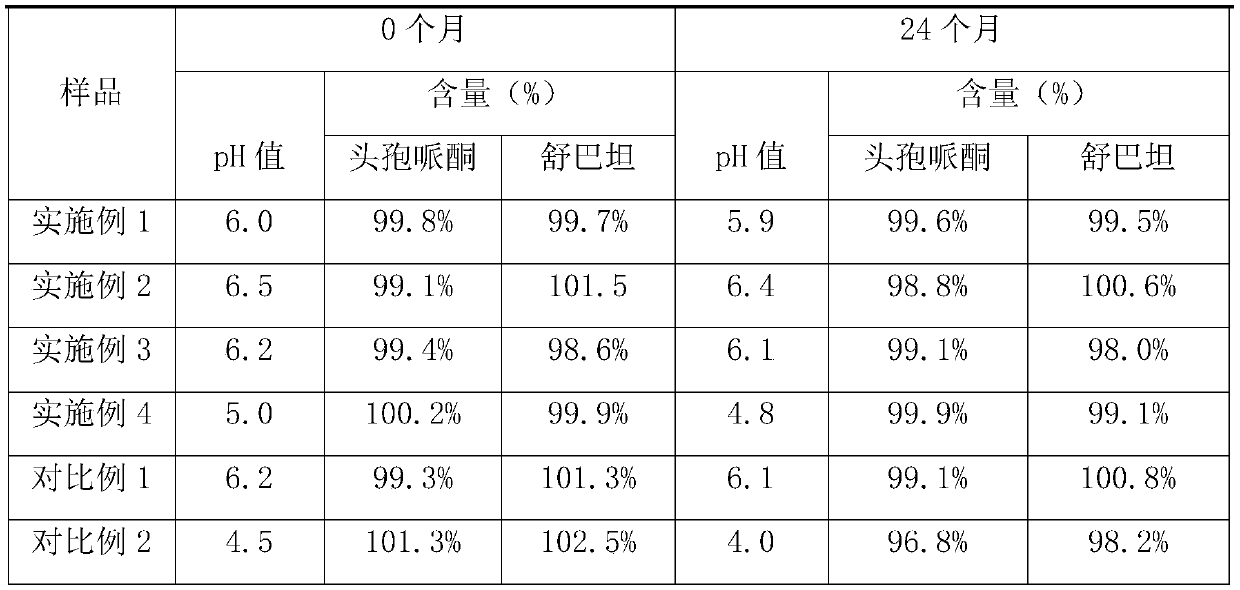
[0027] A cefoperazone sodium sulbactam sodium powder injection for injection, comprising the following components: 1500 g of cefoperazone sodium with a specific rotation of -22°, 900 g of sulbactam sodium, 100 g of glutathione, 30 g of sodium dihydrogen phosphate, and disodium hydrogen phosphate 100g and 30g of sodium chloride, and the pH value of the powder injection is 6.0.
[0028] The preparation method of above-mentioned cefoperazone sodium is as follows:
[0029] (1) Add cefoperazone acid into the acetone solution and stir until completely dissolved, the stirring speed is 100-150r / min, the temperature is controlled at 10-25°C, and then sodium bicarbonate solution and sodium acetate solution are added in turn to adjust the pH Value is 6.5, obtains cefoperazone sodium solution; Wherein, the molar weight of cefoperazone acid is identical with the total molar amount of sodium bicarbonate and sodium acetate, and the mol ratio of sodium bicarbonate and sodium acetate is 5:1; ...
Embodiment 2
[0038] A cefoperazone sodium sulbactam sodium powder injection for injection, comprising the following components: 1000 g of cefoperazone sodium with a specific rotation of -23°, 500 g of sulbactam sodium, 50 g of glutathione, 10 g of sodium dihydrogen phosphate, and disodium hydrogen phosphate 10g and sodium chloride 50g, and the pH value of the powder injection is 6.5.
[0039] The preparation method of above-mentioned cefoperazone sodium is as follows:
[0040](1) Add cefoperazone acid into the acetone solution and stir until completely dissolved, the stirring speed is 100-150r / min, the temperature is controlled at 10-25°C, and then sodium bicarbonate solution and sodium acetate solution are added in turn to adjust the pH Value is 7.0, obtains cefoperazone sodium solution; Wherein, the molar weight of cefoperazone acid is identical with the total molar amount of sodium bicarbonate and sodium acetate, and the mol ratio of sodium bicarbonate and sodium acetate is 8:1;
[004...
Embodiment 3
[0046] A cefoperazone sodium sulbactam sodium powder injection for injection, comprising the following components: 2100 g of cefoperazone sodium with a specific rotation of -22°, 1000 g of sulbactam sodium, 70 g of glutathione, 60 g of sodium dihydrogen phosphate, and disodium hydrogen phosphate 60g and sodium chloride 80g, and the pH value of powder injection is 6.2.
[0047] In this example, the preparation method of cefoperazone sodium and the preparation method of cefoperazone sodium sulbactam sodium powder for injection can refer to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com