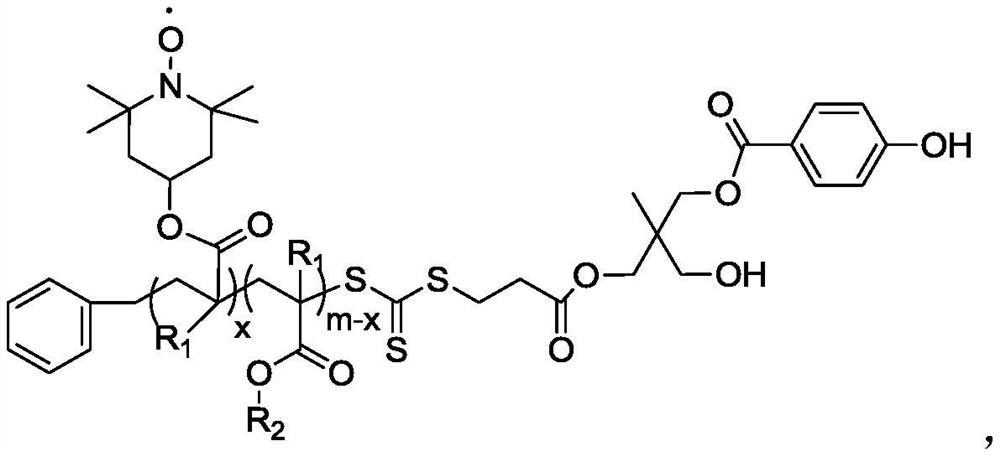
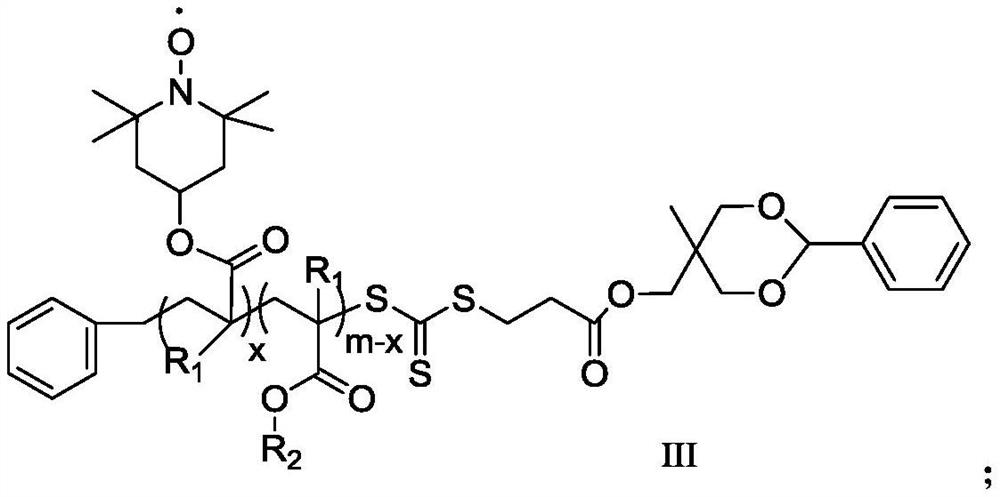
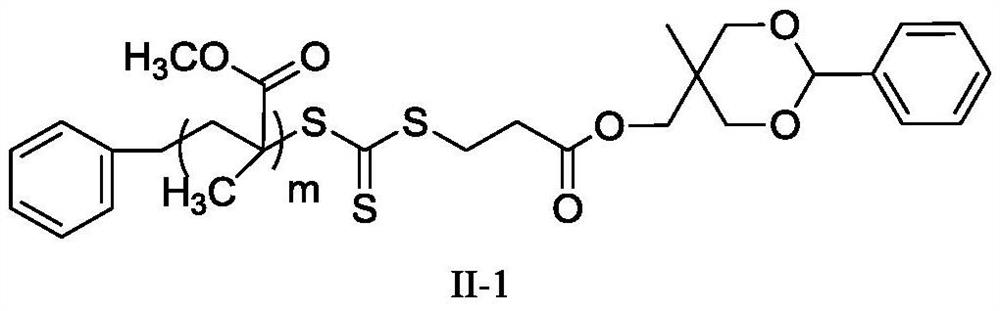
A kind of polymerization inhibitor and its preparation method and application
A technology of polymerization inhibitor and initiator, which is applied in the application field of polymer homogeneous supported polymerization inhibitor and acrylate preparation, can solve the problems of easy entrainment, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) dissolve MHPD and benzyl trithiocarbonate propionic acid in dichloromethane, add catalyst 4-dimethylaminopyridine and N,N-dicyclohexylcarbodiimide, wherein benzyl trithio The molar ratio of carbonate-based propionic acid and MHPD is 1:1, the molar ratio of 4-dimethylaminopyridine and MHPD is 0.1:1, and the molar ratio of N,N-dicyclohexylcarbodiimide and MHPD is 1:1: 1. Under the condition of 20°C, react for 24h, filter after the reaction, concentrate by rotary evaporation, and precipitate twice with diethyl ether to obtain a light yellow solid, which is dried in vacuo to obtain the compound of formula I; The compound of formula I obtained is subjected to infrared analysis, and 1732cm appears -1 ester bond peaks.
[0065] (2) the compound of formula I and methyl methacrylate are dissolved in N,N-dimethylformamide, and the reaction is carried out under the initiation of 2,2'-azobisisobutyronitrile, wherein the initiator and formula I The molar ratio of the compound...
Embodiment 2
[0074] (1) dissolve MHPD and benzyl trithiocarbonate propionic acid in chloroform, add catalyst 4-dimethylaminopyridine and N,N-dicyclohexylcarbodiimide, wherein benzyl trithio The molar ratio of carbonate-based propionic acid and MHPD is 3:1, the molar ratio of 4-dimethylaminopyridine and MHPD is 0.3:1, and the molar ratio of N,N-dicyclohexylcarbodiimide and MHPD is 3:1 1. Under the condition of 50°C, react for 72h, filter after the reaction, concentrate by rotary evaporation, and precipitate twice with diethyl ether as a solvent to obtain a light yellow solid, which is dried in vacuo to obtain the compound of formula I; The obtained compound of formula I was subjected to infrared analysis, and 1737 cm appeared -1 ester bond peaks.
[0075] (2) the compound of formula I and methyl acrylate are dissolved in N,N-dimethylacetamide, and the reaction is carried out under the initiation of 2,2'-azobisisoheptanenitrile, wherein the initiator and the compound of formula I The mola...
Embodiment 3
[0085] (1) dissolve MHPD and benzyl trithiocarbonate propionic acid in solvent chloroform, add catalyst 4-dimethylaminopyridine and N,N-dicyclohexylcarbodiimide, wherein benzyl trisulfide The molar ratio of carbonate-based propionic acid and MHPD is 2:1, the molar ratio of 4-dimethylaminopyridine and MHPD is 0.2:1, and the molar ratio of N,N-dicyclohexylcarbodiimide and MHPD is 2 : 1, under the condition of 25 ℃, react for 48h, filter after the reaction, concentrate by rotary evaporation, and precipitate twice with diethyl ether to obtain a light yellow solid, which is dried in vacuo to obtain the compound of formula I; The obtained compound of formula I was subjected to infrared analysis, and 1725cm appeared -1 ester bond peaks.
[0086] (2) the compound of formula I and ethyl methacrylate are dissolved in N,N-dimethylacetamide, and the reaction is carried out under the initiation of 2,2'-azobisisobutyronitrile, wherein the initiator and formula I The molar ratio of the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com