Mirogabalin sustained-release tablet and preparation method thereof
A technology of sustained-release tablets and benzenesulfonic acid, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve problems such as disease reporting and insufficient treatment, and achieve good analgesic effects. , the effect of improving appearance and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Core layer (per piece):
[0041]
[0042] (2) Composition of film coating solution
[0043] Opadry 02N-650001-CN 15mg
[0044] Embodiment 1——Example 10 Milopalin Besylate Sustained-release Tablet Preparation Technology:
[0045] (1) Grinding the bulk drug to a particle size D 50 Less than 60μm. Pass the auxiliary material through a 40 mesh sieve. The raw and auxiliary materials are weighed according to the prescription amount, and mixed uniformly according to the equal amount incremental method to obtain the premixed powder. Add an appropriate amount of binder to make soft materials, granulate through a 18-mesh sieve, dry on a fluidized bed, and granulate through a 20-mesh sieve. Add the prescribed amount of lubricant and mix well.
[0046] (2) Tablet compression: use a special-shaped die with a size of 12.2mm×6.5mm, and control the hardness to 10-15kg.
[0047] (3) Colored film coating: use Opadry 02N-650001-CN to coat the above-mentioned tablet core, and...
Embodiment 2
[0051] (1) Core layer (per piece):
[0052]
[0053] (2) Composition of film coating solution
[0054] Opadry 02N-650001-CN 15mg
Embodiment 3
[0056] (1) Core layer (per piece):
[0057]
[0058] (2) Composition of film coating solution
[0059] Opadry 02N-650001-CN 15mg
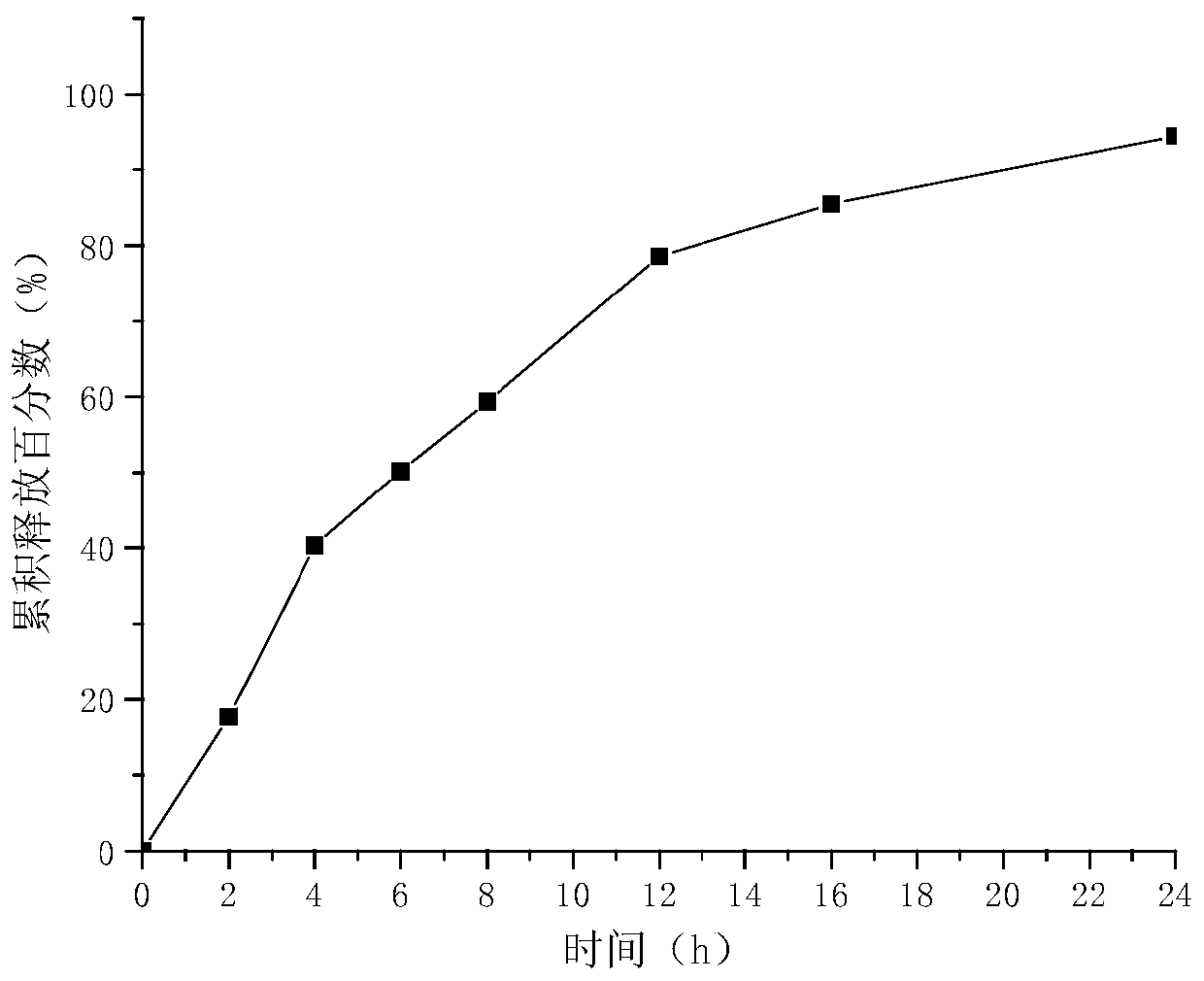
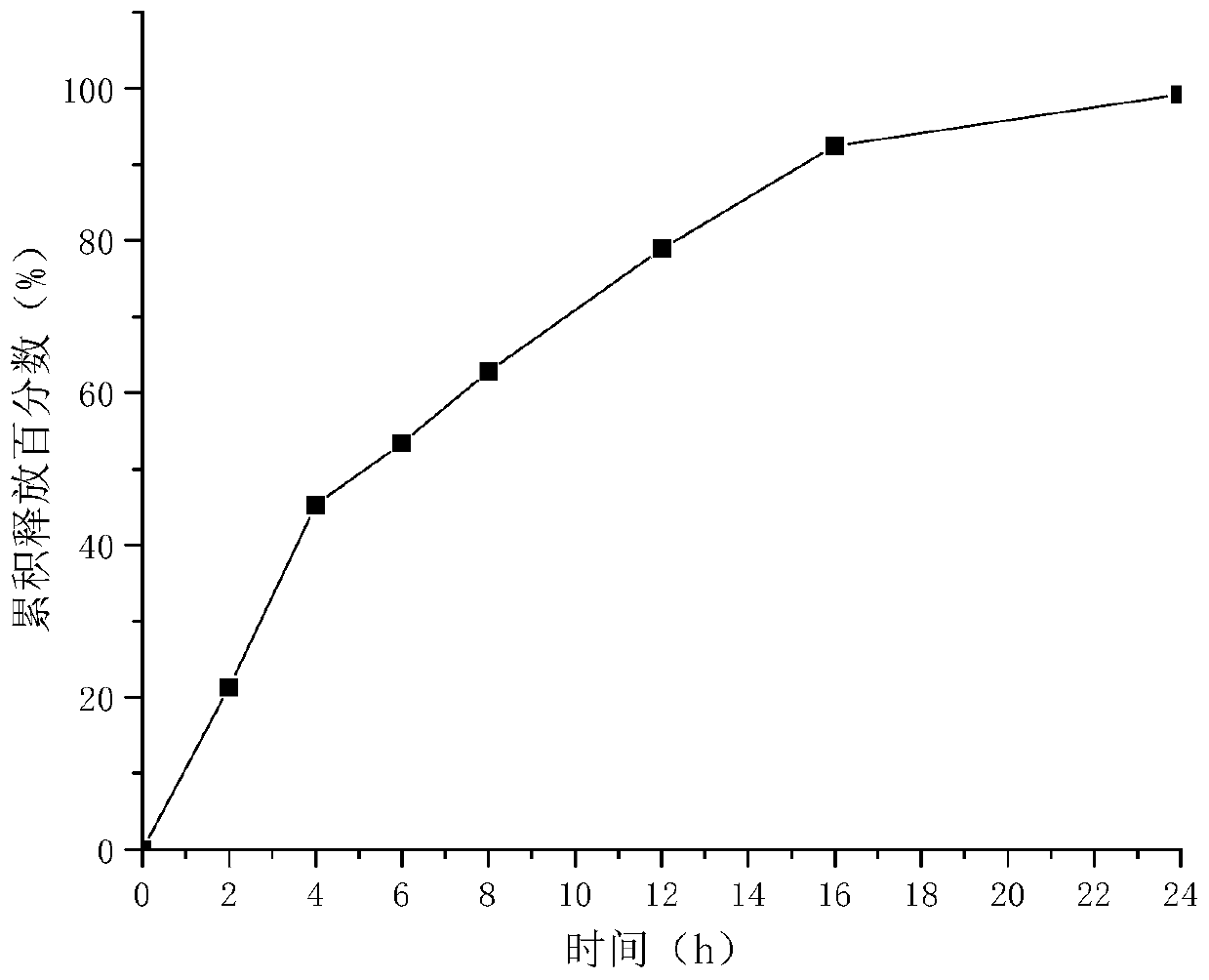
[0060] The release rate of the milobarin besylate sustained-release tablet of embodiment 1 is shown in figure 1 . The release rate of the milobarin besylate sustained-release tablet of embodiment 2 is shown in figure 2 . The release rate of the milobarin besylate sustained-release tablet of embodiment 3 is shown in image 3 . It can be seen that the weight ratio between self-made samples 1, 2, and 3, that is, high-viscosity hypromellose and low-viscosity hypromellose, is 1:0.5, and more than 75% can be released in 12 hours, and The release was complete at 24 hours, indicating that the release curves of the three different specifications of milobarin besylate sustained-release tablets were consistent, and all of them could achieve good sustained-release effects in vitro.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com