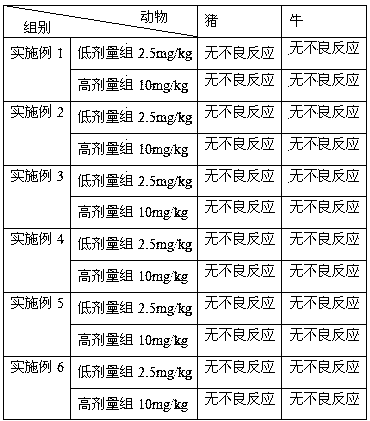
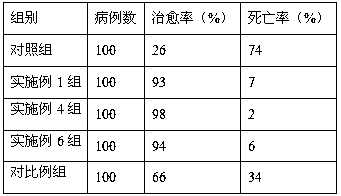
Veterinary tilmicosin premix powder drug
A technology of tilmicosin and premix, which is applied in powder delivery, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of toxic and side effects, inability to cure diseases, etc., and achieve strong tissue penetration and preparation Stable, long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Prescription: every 100g of medicine contains 18g of tilmicosin, 1.5g of trimethoprim lactate, and the balance is anhydrous glucose.
[0018] Preparation:
[0019] (1) Grind and sieve the tilmicosin, trimethoprim lactate, and anhydrous glucose in the prescription amount, respectively, and set aside;
[0020] (2) Weigh the two raw materials of tilmicosin and trimethoprim lactate, add the same amount of anhydrous glucose as the sum of the two raw materials, and pre-mix for 15 minutes according to the dispersion method;
[0021] (3) Then add the remaining anhydrous glucose and mix thoroughly for 40 minutes, and mix well.
Embodiment 2
[0023] Prescription: every 100g of medicine contains 18g of tilmicosin, 2.5g of trimethoprim lactate, and the balance is anhydrous glucose.
[0024] The preparation method is the same as in Example 1.
Embodiment 3
[0026] Prescription: every 100g of medicine contains 19g of tilmicosin, 1.8g of trimethoprim lactate, and the balance is anhydrous glucose.
[0027] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com