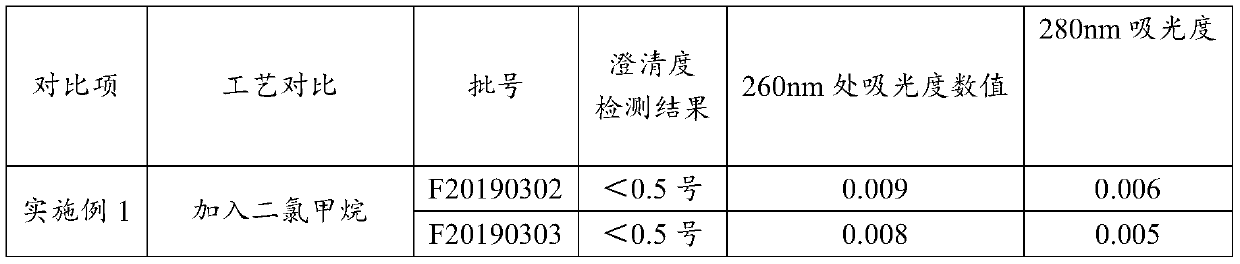
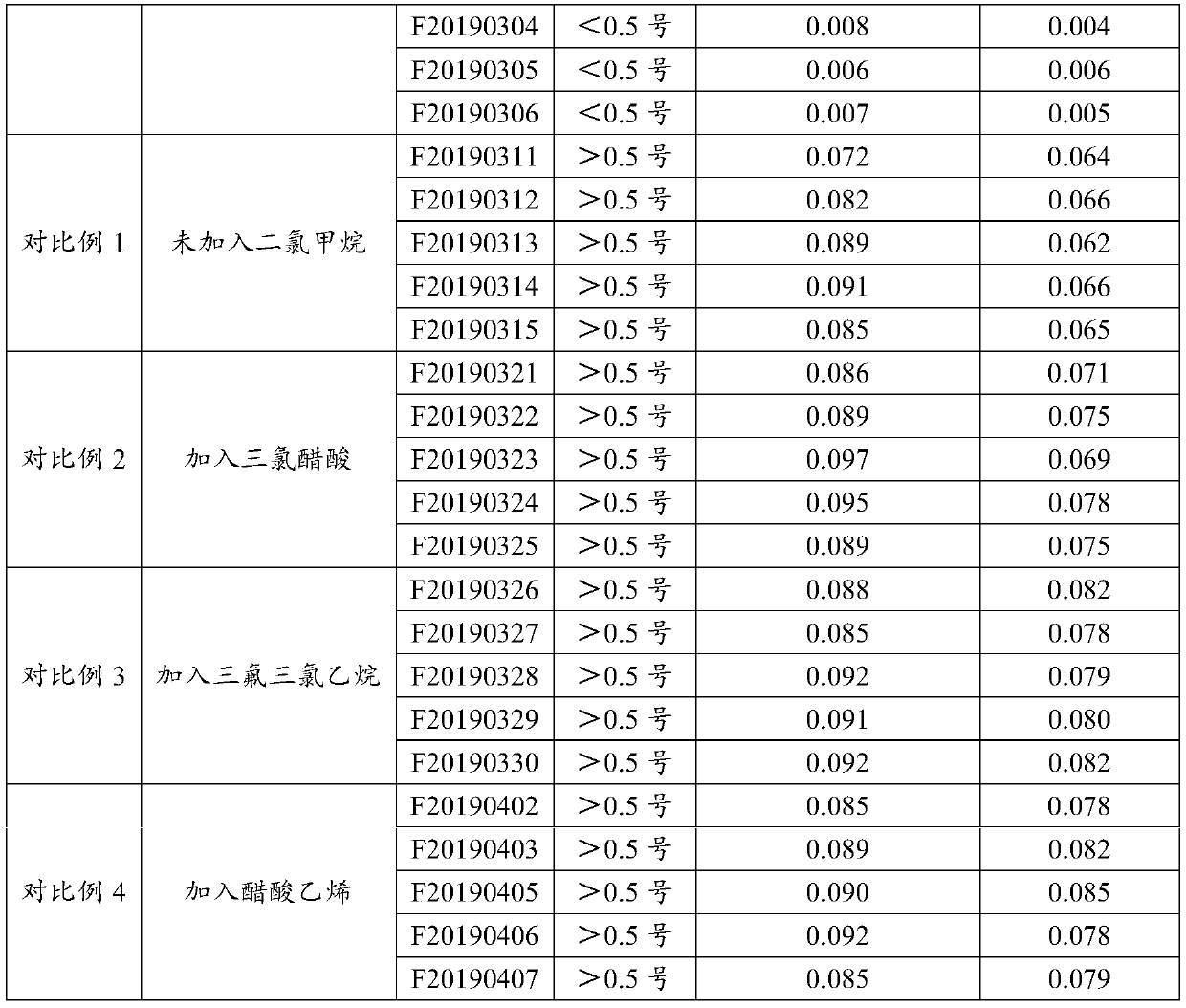
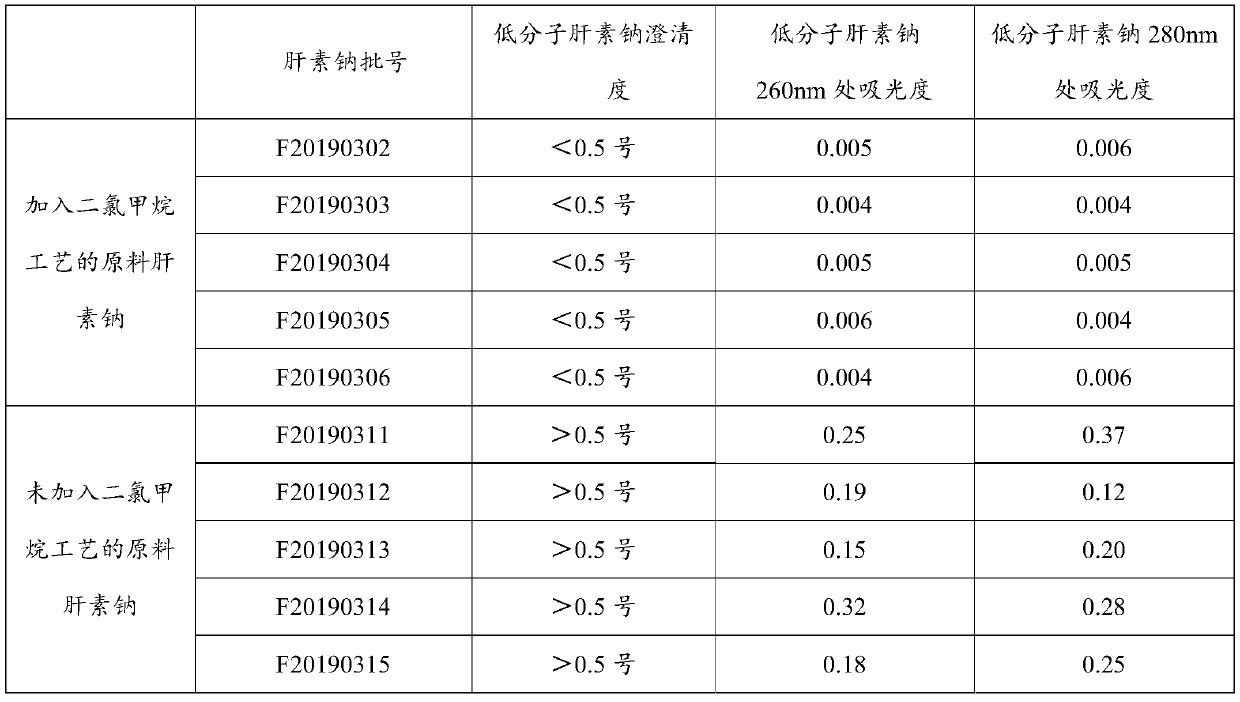
Heparin sodium high in clarity
A heparin sodium, high-purity technology, applied in the direction of blood diseases, extracellular fluid diseases, non-central analgesics, etc., can solve the problems of reduced insoluble particles, residual protein and nucleic acid, etc., to achieve reduced insoluble particles, low cost, The effect of simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A preparation process for high-purity heparin sodium, specifically comprising the following steps:
[0040] 1. Enzymatic deproteinization: dilute the crude heparin sodium with purified water to a 10% (w / v) concentration solution, raise the temperature to 45.0°C, adjust the pH value to 7-9, add the dissolved 2709 protease, and keep the temperature and pH, keep stirring for 6 hours, then add calcium chloride solution, keep stirring, and filter with plate and frame filter;
[0041]2. Oxidation: adjust the temperature of the filtrate to room temperature °C, adjust the pH value of the feed liquid to 10-11 with 4mol / L sodium hydroxide solution, and add 30% of the feed liquid volume to 1% of the feed liquid volume under the condition of maintaining pH 10-11. For hydrogen peroxide oxidation, check the pH change of the feed liquid every half hour. If the pH is lower than 10, adjust it to the range of 10-11 in time, and add 0.5% hydrogen peroxide. The overall hydrogen peroxide ad...
Embodiment 2
[0048] A preparation process for high-purity heparin sodium, specifically comprising the following steps:
[0049] 1. Enzymatic deproteinization: Dilute the crude heparin sodium with purified water to a solution with a concentration of 5% (w / v), raise the temperature to 55.0°C, adjust the pH value to 7-9, add dissolved 2709 protease, and keep the temperature and pH, keep stirring for 6 hours, then add calcium chloride solution, keep stirring, and filter with plate and frame filter;
[0050] 2. Oxidation: adjust the temperature of the filtrate to room temperature °C, adjust the pH value of the feed liquid to 10-11 with 4mol / L sodium hydroxide solution, and add 30% of the feed liquid volume to 1% of the feed liquid volume under the condition of maintaining pH 10-11. For hydrogen peroxide oxidation, check the pH change of the feed liquid every half hour. If the pH is lower than 10, adjust it to the range of 10-11 in time, and add 0.5% hydrogen peroxide. The overall hydrogen perox...
Embodiment 3
[0057] A preparation process for high-purity heparin sodium, specifically comprising the following steps:
[0058] 1. Enzymatic deproteinization: dilute the crude heparin sodium with purified water to a solution with a concentration of 15% (w / v), raise the temperature to 35.0°C, adjust the pH value to 7-9, add dissolved 2709 protease, and keep the temperature and pH, after stirring for 6 hours, add calcium chloride solution, continue stirring, and filter with a plate and frame filter;
[0059] 2. Oxidation: adjust the temperature of the filtrate to room temperature °C, adjust the pH value of the feed liquid to 10-11 with 4mol / L sodium hydroxide solution, and add 30% of the feed liquid volume to 1% of the feed liquid volume under the condition of maintaining pH 10-11. For hydrogen peroxide oxidation, check the pH change of the feed liquid every half hour. If the pH is lower than 10, adjust it to the range of 10-11 in time, and add 0.5% hydrogen peroxide. The overall hydrogen pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com