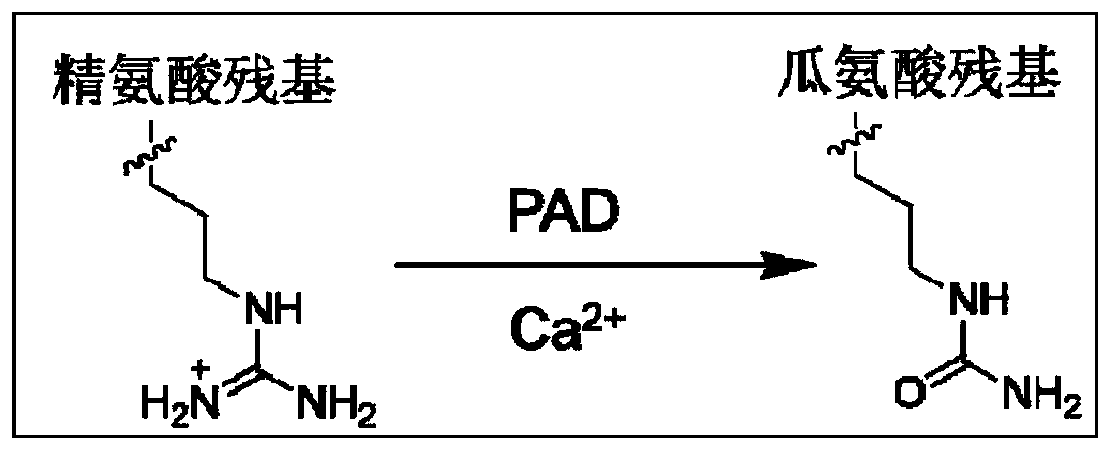
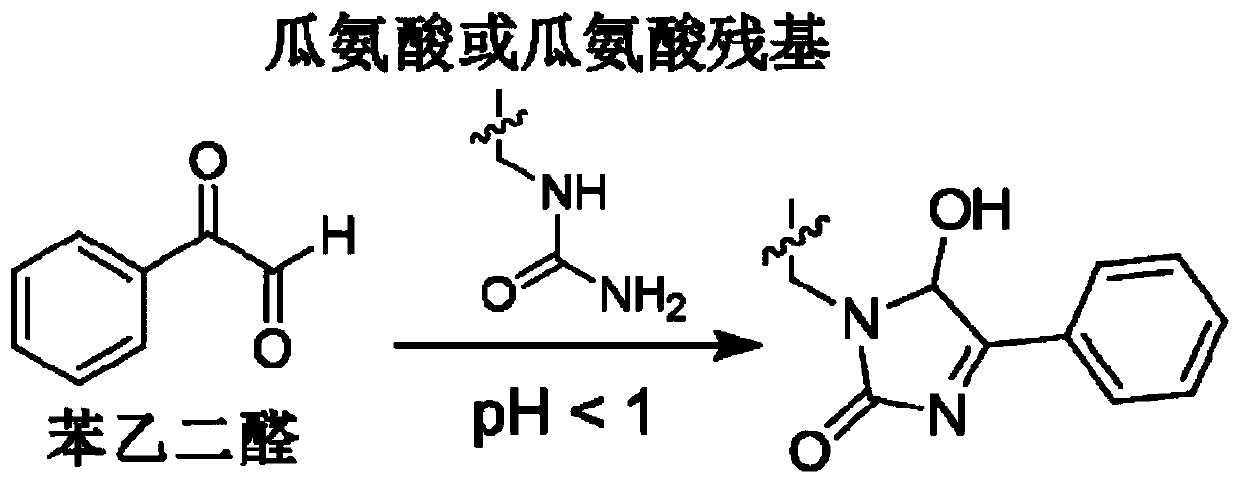
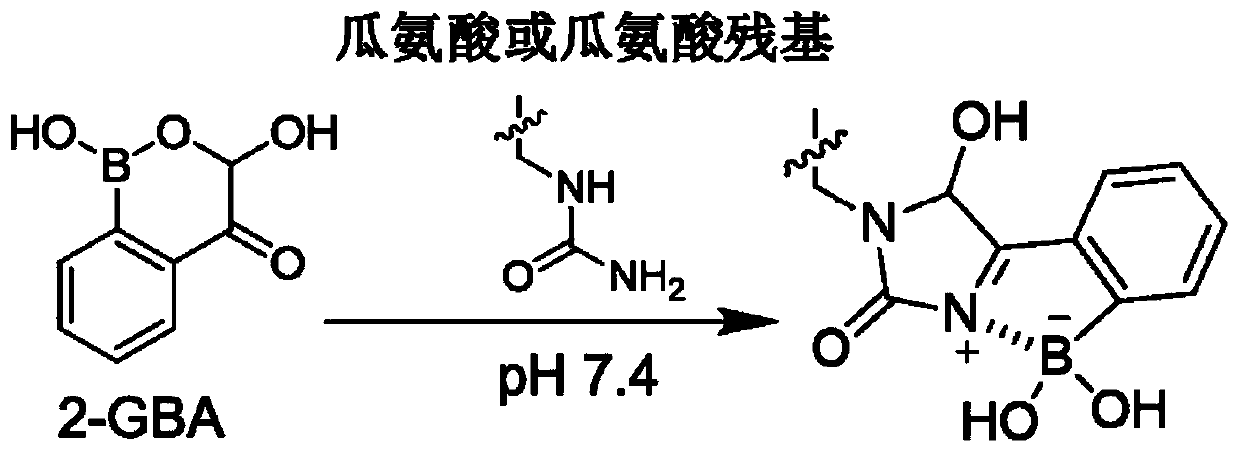
Citrulline probe compound and application thereof
A compound, citrulline technology, applied in the field of citrulline probe compounds, can solve problems such as inability to apply cells, poor citrulline detection effect, and hindering probe application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of compound 2-GBA
[0049]
[0050] Dissolve 2-acetylphenylboronic acid (100 mg, 0.6 mmol) in 10 mL DMSO / H 2 In a mixed solvent of O (1:1), iodine (228mg, 0.9mmol) was then added, and heated to 100°C for 3 hours. Stirring was stopped, the reaction was removed, quenched with 0.2M aqueous sodium thiosulfate solution, extracted three times with 40 mL ether, the organic phases were combined, washed once with saturated aqueous NaCl solution, and dried over anhydrous sodium sulfate. The solvent was spin-dried on a rotary evaporator to obtain a crude yellow solid. Recrystallized from water to obtain 56 mg of pure yellow product with a yield of 51%. NMR: 1 H NMR (400MHz,D 2 O / CD 3 CN=5:1)δ / ppm:7.97-7.95(d,J=7.6Hz,1H),7.86-7.85(d,J=7.2Hz,1H),7.80-7.76(m,1H),7.71-7.67 (m,1H),5.62(s,1H); 1 H NMR (400MHz,d 6 -DMSO)δ / ppm: 9.23 (s, 1H), 7.91-7.87 (m, 2H), 7.79-7.69 (m, 2H), 7.64-7.62 (d, J=7.6Hz, 1H), 5.42-5.40 ( d,J=7.6Hz,1H); 1 H NMR (400MHz, CD 3 OD)δ / ppm: ...
Embodiment 2
[0053] The synthetic route of the compound shown in 2-GBA-Biotin is as follows:
[0054]
[0055] The preparation method of the compound shown in 2-GBA-Biotin comprises the following steps:
[0056] (1) Preparation of compound 2:
[0057] Add 2-4 dihydroxyacetophenone (500mg, 3.28mmol), anhydrous potassium carbonate (681mg, 4.92mmol) and 1,2-dibromoethane (924mg, 4.92mmol) into 25mL of acetonitrile and heat to 40 °C, stirred for 12 hours. Stop heating, let it cool to room temperature, add 50 mL of water, and then extract with dichloromethane (50 mL×3), collect and combine the organic phases. followed by H 2 O. Wash once with saturated NaCl aqueous solution, then add anhydrous NaCl 2 SO 4 After drying, filtering and rotary evaporation under reduced pressure, the crude product was obtained as a white powdery solid. Separation and purification by 200-300 mesh silica gel column chromatography [eluent: V (petroleum ether): V (ethyl acetate) = 15: 1] to obtain 463 mg of pur...
Embodiment 3
[0073] 1. The reaction of the compound shown in 2-GBA with citrulline:
[0074] The compound shown in 2-GBA or phenylglyoxal and L-citrulline are reacted under neutral conditions, and the reactivity is tested, and the reaction conditions are: 2-GBA or phenylglyoxal (1.1 equivalents) and L-citrulline (1.0 equiv) in 1X PBS and CH 3 Mixed solution of CN (PBS and CH 3 The volume ratio of CN is: 3:1), reacted at 37°C for 3h, and tested the 1H NMR spectra of the reaction products (2-GBA+L-citrulline and phenylglyoxal+L-citrulline), and the 1H NMR spectrum of pure L-citrulline, see Figure 5 . from Figure 5 It can be seen that in 2-GBA+L-citrulline, the signal peak of L-citrulline is gradually disappearing, and two new peaks appear at the same time, suggesting that a chemical reaction occurs. And under the same conditions, there is no change in the 1H NMR comparison spectrum of phenylglyoxal and citrulline reaction, which is also consistent with existing reports, indicating tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com