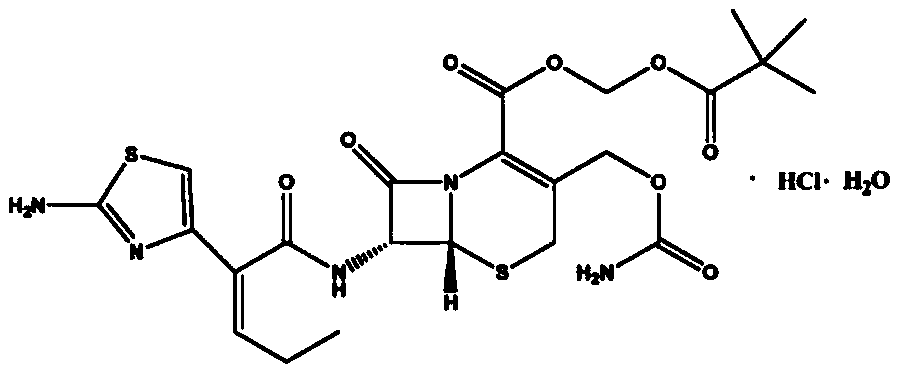
Cefcapene pivoxil hydrochloride granule and preparation method thereof
A technology for cefcapine hydrochloride and cefcapine hydrochloride, which is applied in the directions of anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc. problems such as low rate, to achieve the effect of simple formula and process, high yield of finished product and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
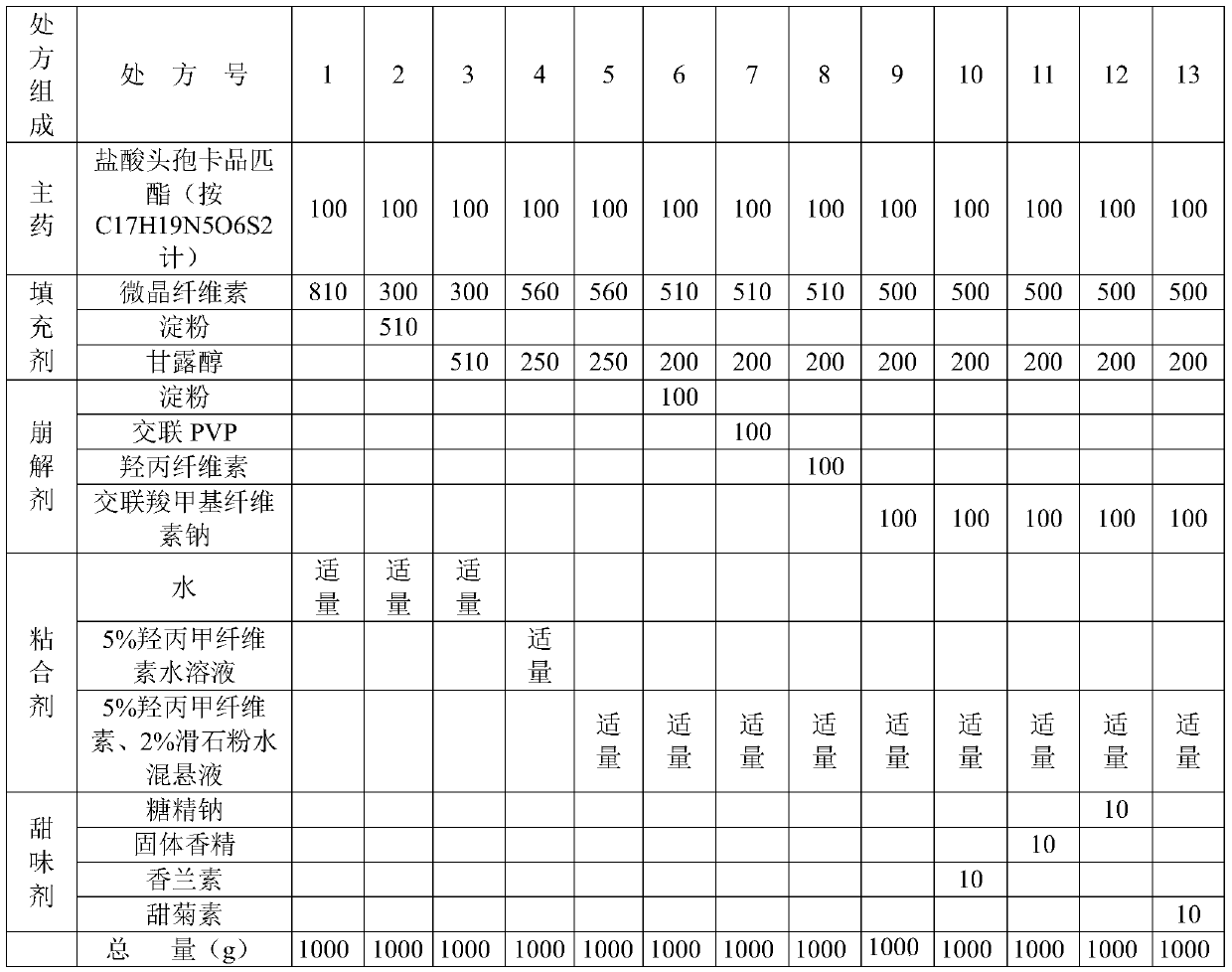
[0024] Experimental Example 1: Screening and investigation of fillers, disintegrants, binders, and sweeteners in the prescription, the main inspection items: preparation formability, appearance, hardness, etc. of the drug-containing pellets, the formulation screening is shown in the table 1.
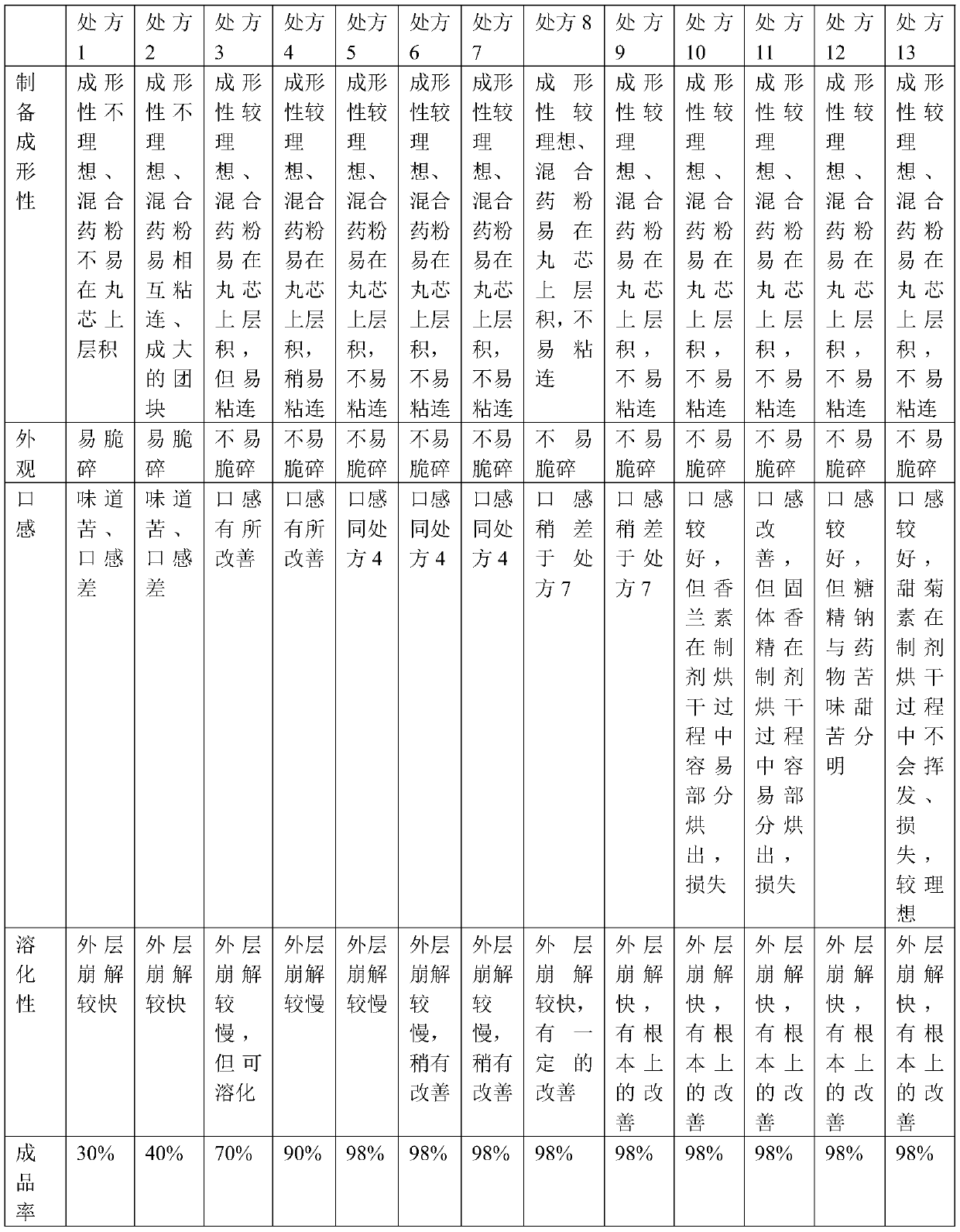
[0025] Inspection items: preparation formability, appearance, particle size, solubility, taste, etc. of drug-containing pellets.
[0026] Examination method: Refer to Appendix ⅠN of the second volume of "Chinese Pharmacopoeia" 2010 edition.
[0027] Preparation formability, appearance: visual inspection; particle size, solubility: refer to "Chinese Pharmacopoeia" 2010 edition, appendix I N; taste: tasting. The experimental results of each prescription are shown in Table 2.
[0028] Table 1 Prescription Screening
[0029]
[0030] Note 1: Cefcarpine pivoxil hydrochloride is produced by Zhejiang Huafang Pharmaceutical Co., Ltd. 17 h 19 N 5 o 6 S 2 100g is equivalent to 140g raw ...
experiment example 2
[0049] Experimental example 2: Influencing factors Test method: Take this product, remove the outer packaging, put it in a suitable open container, and conduct the following tests:
[0050] (1) High temperature test Take this product, put it in a suitable clean container, store it at 60°C for 10 days, take samples on the 5th and 10th day, and test according to the stability investigation items of this product. The results are shown in Table 3.
[0051] (2) High humidity test Take this product, put it in a closed container with constant humidity, and place it at 25°C for 10 days under the conditions of relative humidity 90% ± 5% and 75% ± 5%, respectively, on the 5th and 10th day Sampling was carried out according to the stability investigation project of this product, and the measurement results are shown in Table 3.
[0052] (3) Illumination test Take this product, put it in a light box device equipped with a fluorescent lamp, and place it under the condition of an illuminati...
experiment example 3
[0056] Experimental example 3: Accelerated test In accordance with the relevant provisions of the Chinese Pharmacopoeia 2010 edition of the drug stability test guidelines, the accelerated test of this product is packaged in the market, under the conditions of a temperature of 40°C ± 2°C and a relative humidity of 75% ± 5%. carried out for 6 months. Samples were taken at the end of the first month, second month, third month, and sixth month of the test period, and tested according to the stability inspection items of this product. The results are shown in Table 4.
[0057] Table 4 Accelerated test results (40°C±2°C, RH75%±5%)
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com