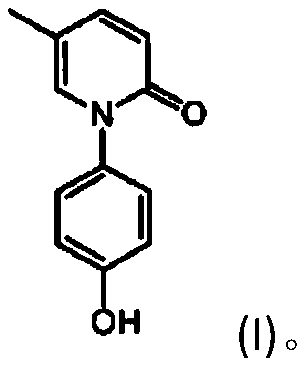
Preparation method of hydronidone
A technology of oxynidone and pyridone, which is applied in the field of preparation of oxynidone, can solve the problems of cumbersome sewage and waste treatment, easy formation of highly toxic gas, expensive reagents, etc., and achieves suitable for large-scale production, high yield, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
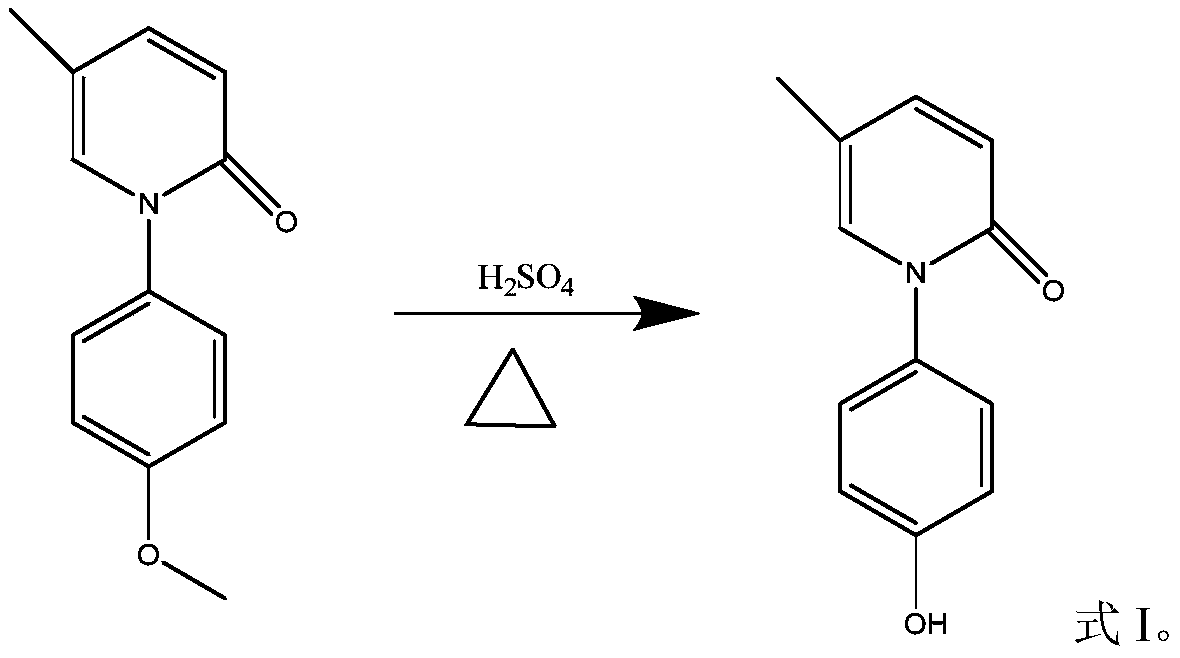
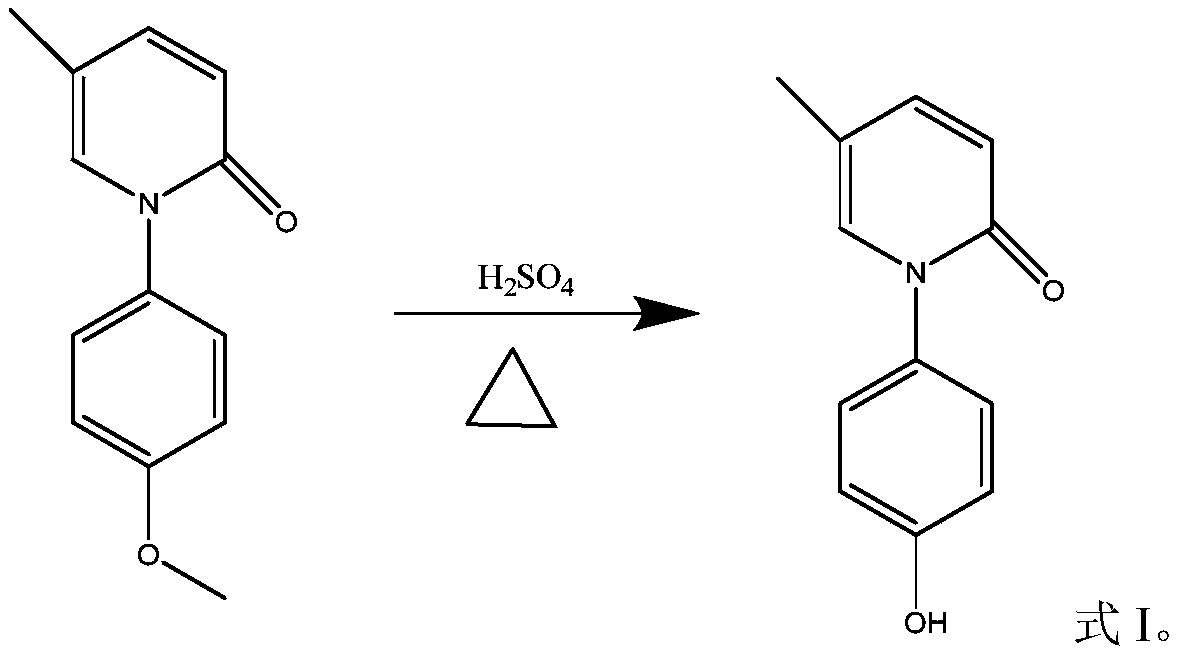
[0031] Reaction equation:
[0032]
[0033] Feed amount and feed ratio
[0034] Feeding amount and charging ratio of table 2 embodiment 1
[0035]
[0036] 1. Preparation of crude compound of formula I
[0037] 1.1 get the sulfate compound of formula I
[0038] Put 22 kg of N-(4-methoxyphenyl)-5-methyl-2-pyridone (compound of formula II) into the reactor, then add 180 kg of 65% sulfuric acid under stirring, and start to heat up to strong reflux after heating , the external temperature is controlled at 165°C, and the internal temperature is controlled at 148°C-150°C. After about 4 hours, there is no raw material point in the spot plate test, and the raw material content is lower than 6% in the LC test. Stop heating, cool to about 60°C, and add drinking water 100 kg, continue to cool, crystallize at 5°C to 10°C for 2 hours, filter, wash the filter cake three times with 15 kg of ethyl acetate (three equal parts), put the filter cake in an evaporating dish and dry it to...
Embodiment 2
[0047] Feed amount and feed ratio
[0048] Feeding amount and charging ratio of table 3 embodiment 2
[0049]
[0050]
[0051] 2.1 Preparation of crude compound of formula I
[0052] Put 11 kilograms of N-(4-methoxyphenyl)-5-methyl-2-pyridone (compound of formula II) into the reactor, then add 180 kilograms of 50% sulfuric acid under stirring, and start to heat up to strong Reflux, the reaction temperature is controlled at 120°C to 125°C, after about 24 hours, there is no raw material point in the TLC test, and the raw material content is less than 6% in the LC test, stop heating, cool to about 60°C, add 20 kg of water, continue cooling, 5°C Crystallize at ~10°C for 2 hours, filter, wash the filter cake three times with 7.5 kg of ethyl acetate, and put the filter cake in an evaporating dish to dry. Get about 11 kilograms of the sulfate compound of formula I.
[0053]Post-treatment (neutralization and crystallization, beating and refining, refining decolorization) i...
Embodiment 3
[0055] Feed amount and feed ratio
[0056] Feeding amount and charging ratio of table 4 embodiment 3
[0057]
[0058] 3.1 Preparation of crude compound of formula I
[0059] Put 11 kilograms of N-(4-methoxyphenyl)-5-methyl-2-pyridone (compound of formula II) into the reactor, then add 62.5 kilograms of 80% sulfuric acid under stirring, and start to heat up to strong after heating. Reflux, the reaction temperature is controlled at 165°C to 170°C. After about 2 hours, there is no raw material point in the TLC test, and the raw material content is less than 6% in the LC test. Stop heating, cool to about 60°C, add 60 kg of water, continue to cool, 5°C Crystallize at ~10°C for 2 hours, filter, wash the filter cake three times with 7.5 kg of ethyl acetate, and put the filter cake in an evaporating dish to dry. Get about 10 kilograms of the sulfate compound of formula I.
[0060] Post-treatment (neutralization and crystallization, beating and refining, refining decolorizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com