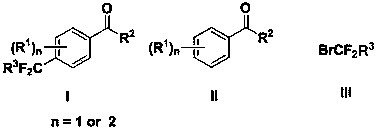
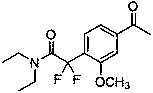
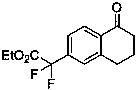
Synthesis method of difluoroalkyl substituted aromatic ketone compound under photocatalysis
The technology of a difluoroalkyl group and a synthesis method is applied in the field of synthesis of difluoroalkyl substituted aromatic ketone compounds, which can solve the problem of high temperature and achieve the effects of simple operation, wide substrate range and high regioselectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 2-(4-Acetylphenyl)-2,2-difluoroethyl acetate (I-a)
[0026]
[0027] Add compound (II) acetophenone (48.1mg, 0.4mmol), Ir(ppy) into a 50mL round-bottomed flask equipped with a magnetic stir bar 3 (13mg, 0.02mmol), 1,10-phenanthroline (36mg, 0.2mmol), cesium carbonate (521mg, 1.6mmol) and ethyl bromodifluoroacetate (162.4mg, 0.8mmol), to the mixture was added normal Heptane (5mL), the mixture was irradiated with 3W blue light, and the reaction was stirred at 25°C for 24 hours. The reaction mixture was washed with saturated brine. The mixture was extracted with ethyl acetate, and the combined organic layer was washed with anhydrous Mg 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using n-hexane / ethyl acetate to obtain 73.2 mg of product with a yield of 75.5% and an HPLC purity of 97.9%.
Embodiment 2
[0028] Example 2 2,2-Difluoro-2-(4-(4-methylbenzoyl)phenyl) ethyl acetate (I-b)
[0029]
[0030] In a 50 mL round bottom flask equipped with a magnetic stir bar, compound (II) phenyl(p-tolyl)methanone (78.5mg, 0.4mmol), rhodamine 6G (9.6mg, 0.02mmol), 2,2'- Bipyridine (31.2mg, 0.2mmol), sodium acetate (98.4mg, 1.2mmol) and ethyl bromodifluoroacetate (242.4mg, 1.2mmol), to the mixture was added n-hexane (5mL), the mixture in 3W Blue light was irradiated, the reaction was stirred at 30°C for 30 hours, and the reaction mixture was washed with saturated brine. The mixture was extracted with ethyl acetate, and the combined organic layer was washed with anhydrous Mg 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using n-hexane / ethyl acetate to obtain 101.4 mg of the product with a yield of 79.6% and an HPLC purity of 98.2%.
Embodiment 3
[0031] Example 3 2-(4-Benzoylphenyl)-2,2-difluoroethyl acetate (I-c)
[0032]
[0033] Add compound (II) benzophenone (72.9mg, 0.4mmol), Ir(ppy) into a 50mL round-bottomed flask equipped with a magnetic stir bar 3 (19.6mg, 0.03mmol), 2,2'-bipyridine (31.2mg, 0.2mmol), potassium carbonate (110.4mg, 0.8mmol) and ethyl bromodifluoroacetate (323.2mg, 1.6mmol), add to the mixture DMSO (5mL) was added to the mixture, the mixture was irradiated with 3W blue light, and the reaction was stirred at 35°C for 18 hours. The reaction mixture was washed with saturated brine. The mixture was extracted with ethyl acetate, and the combined organic layer was washed with anhydrous Mg 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using n-hexane / ethyl acetate to obtain 94 mg of the product with a yield of 77.2% and an HPLC purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com