Micro-channel synthesis method of dinitrobenzene
A synthesis method and technology of dinitrobenzene, applied in the direction of nitro compound preparation, organic chemistry, etc., to achieve process safety and reduce the effect of possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
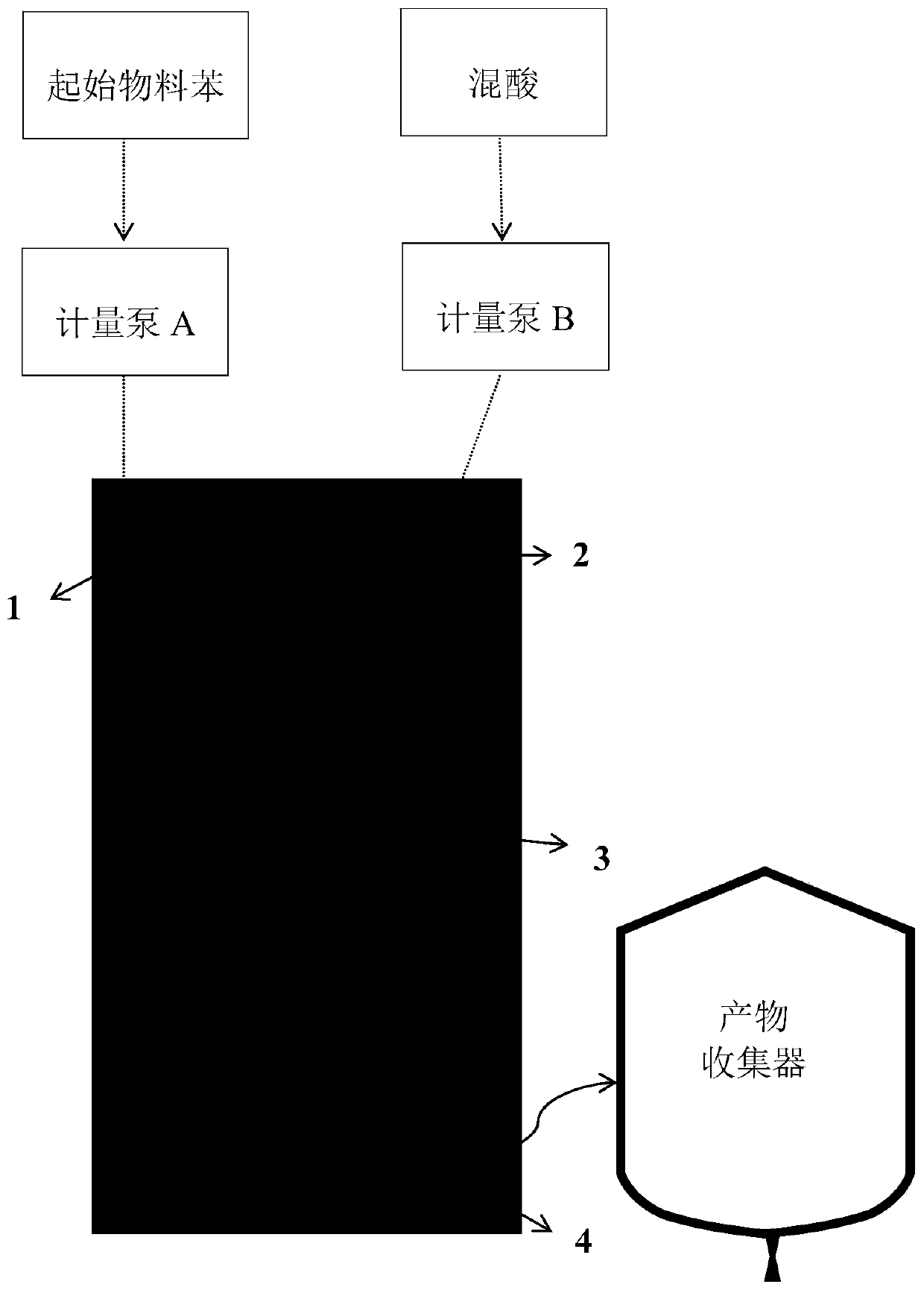
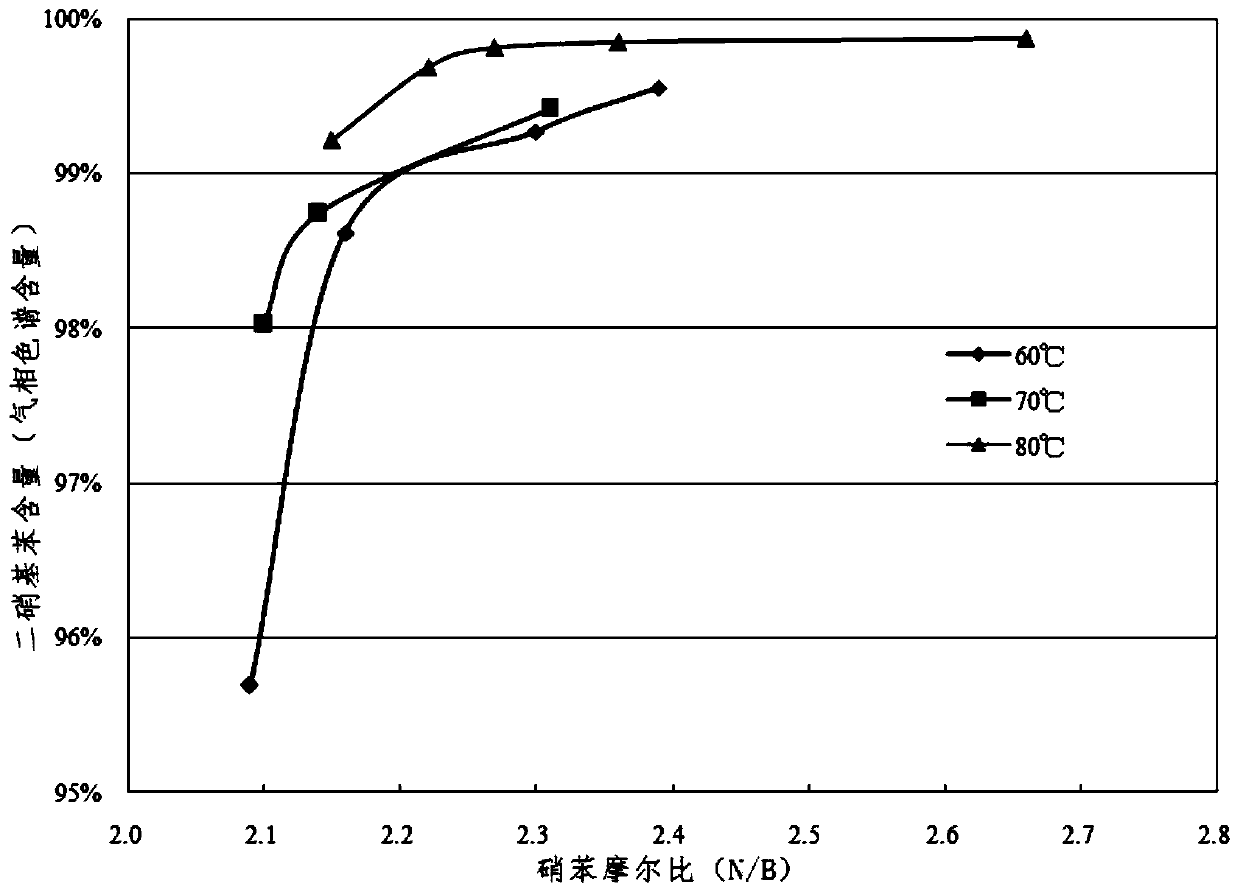
[0044] Fuming nitric acid (≥98%) and concentrated sulfuric acid (≥98%) are used to prepare a mixed acid with a molar ratio of fuming nitric acid and concentrated sulfuric acid of 0.5 and a water concentration of 3%. During the preparation of mixed acid, the temperature can be controlled below 25°C. Under normal temperature conditions, the mixed acid and benzene are continuously pumped into the micro-reactor by the micro-advection pump and react in the micro-reaction channel, and the reaction temperatures are 60°C, 70°C and 80°C respectively. Control the molar ratio of nitric acid and benzene to be ≥ 2.0 and ≤ 2.4. The specific conditions of the microreactor reaction are shown in Table 1.
[0045] Table 1
[0046]
[0047] The reaction product flows out of the reactor continuously, enters the collector and maintains the same reaction temperature as the microreactor for 1 hour, then the reaction material enters the separator and adds an appropriate amount of ice water to te...
Embodiment 13~24
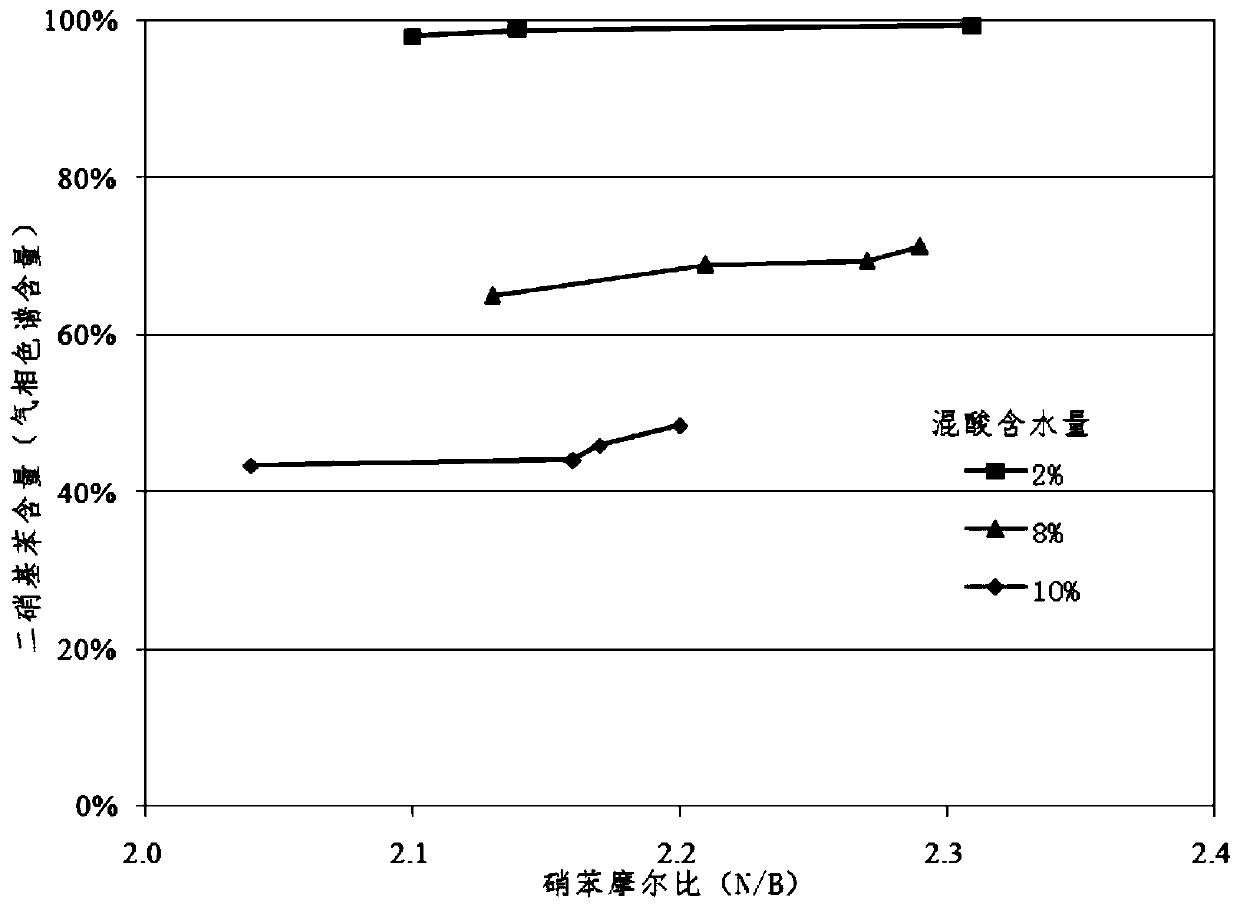
[0050] The process and the reactor used are the same as in Examples 1-5, the molar ratio of nitric acid to sulfuric acid is 0.5, the water content in the mixed acid is 2%, 8% and 10% respectively, the molar ratio of nitric acid to benzene is ≥2.0, and ≤2.4, micro-reaction The receiver and collector were operated at 70°C. The reaction conditions of the microreactor are shown in Table 2. The implementation results are attached image 3 Show.
[0051]
[0052] From image 3 It can be seen that with the increase of N / B, the content of dinitrobenzene increases, which is beneficial to the nitration reaction. The water content in the mixed acid increases, the nitration ability of the mixed acid decreases, and the content of dinitrobenzene decreases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com