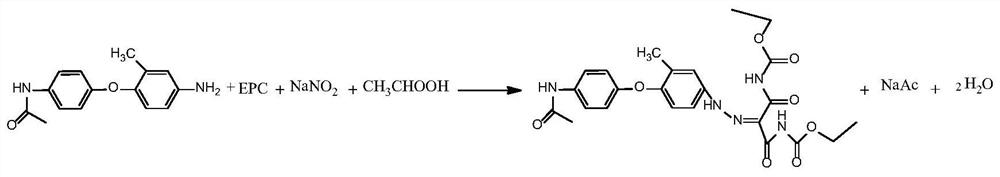
Preparation method of anticoccidial veterinary drug samizuril
An anti-coccidiosis and samide technology, applied in the direction of organic chemistry, can solve the problems of being unsuitable for industrial production, limiting the application prospects of drugs, and complicated post-processing procedures, and achieving shortened overall reaction steps, low cost, and high atom utilization. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The method for preparing the anticoccidial veterinary drug samizuril comprises the following steps:
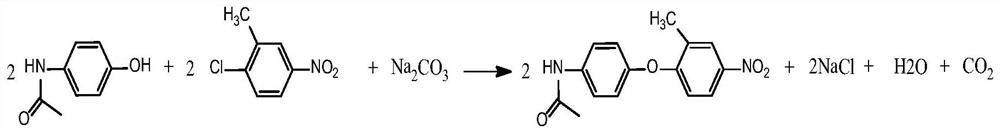
[0035] Such as figure 1 Shown, step 1 condensation reaction: in 250ml four-necked bottle, add organic solvent I DMF 120g, basic acid-binding agent sodium carbonate 26.33g, with 2-chloro-5-nitrotoluene 35.52g, acetaminophen 30g As a raw material, the mol ratio of paracetamol and 2-chloro-5-nitrotoluene is 1:1.05; the mol ratio of paracetamol and alkaline acid-binding agent is 1:1.2; Under the condition of 130°C, a condensation reaction occurs, and the reaction is kept for 5 hours until the reaction is complete, and then separated to obtain a condensation product (structure 1); the yield of this reaction is 90.20%.
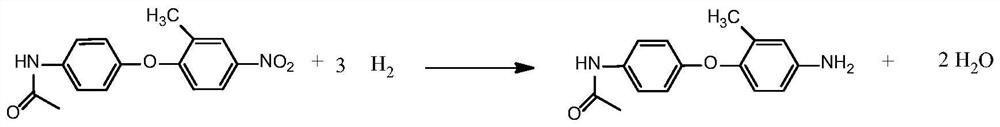
[0036] Such as figure 2 As shown, step 2 reduction reaction: In a 250ml autoclave, add organic solvent II methanol 150g, condensation product (structure one) 50g in methanol, add Raney nickel catalyst 2.5g, condensation product in step 1 (structure one) T...
Embodiment 2
[0042] The method for preparing the anticoccidial veterinary drug samizuril comprises the following steps:
[0043] Such as figure 1 Shown, step 1 condensation reaction: in 250ml four-necked bottle, add organic solvent I DMSO 120g, basic acid-binding agent potassium carbonate 31.47g, with 2-chloro-5-nitrotoluene 35.52g, acetaminophen 30g As a raw material, the mol ratio of paracetamol and alkaline acid-binding agent is 1:1.1, and the mol ratio of paracetamol and 2-chloro-5-nitrotoluene is 1:1.05; Under the condition of 150°C, a condensation reaction occurs, and the reaction is kept for 4 hours until the reaction is complete, and then separated to obtain a condensation product (structure 1); the yield of this reaction is 91.50%.
[0044] Such as figure 2 As shown, step 2 reduction reaction: In a 250ml autoclave, add organic solvent II ethanol 150g, condensation product (structure one) 50g in methanol, add Raney nickel catalyst 2.0g, condensation product (structure one) and R...
Embodiment 3
[0050] The method for preparing the anticoccidial veterinary drug samizuril comprises the following steps:
[0051] Such as figure 1 As shown, step 1 condensation reaction: In a 250ml four-necked bottle, add organic solvent I DMAC120g, alkaline acid-binding agent sodium hydroxide 12.42g, 2-chloro-5-nitrotoluene 37.21g, paracetamol 30g As a raw material, the mol ratio of paracetamol and 2-chloro-5-nitrotoluene is 1:1.1; the mol ratio of paracetamol and alkaline acid-binding agent is 1:1.5; Under the condition of 140°C, a condensation reaction occurs, and the reaction is kept for 5 hours until the reaction is complete, and then separated to obtain a condensation product (structure 1); the yield of this reaction is 91.35%.
[0052] Such as figure 2 As shown, step 2 reduction reaction: In a 250ml autoclave, add organic solvent II toluene 150g, condensation product (structure one) 50g in methanol, add Raney nickel catalyst 10.0g, condensation product (structure one) and Raney T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com