Construction method and fermentation process of engineering bacteria for producing artemisinin precursor
A technology of artemisinin and construction method, which is applied to the construction of recombinant bacteria for producing artemisinin precursor and the field of fermentation technology, and can solve the problems of high cost, price fluctuation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Strain optimization
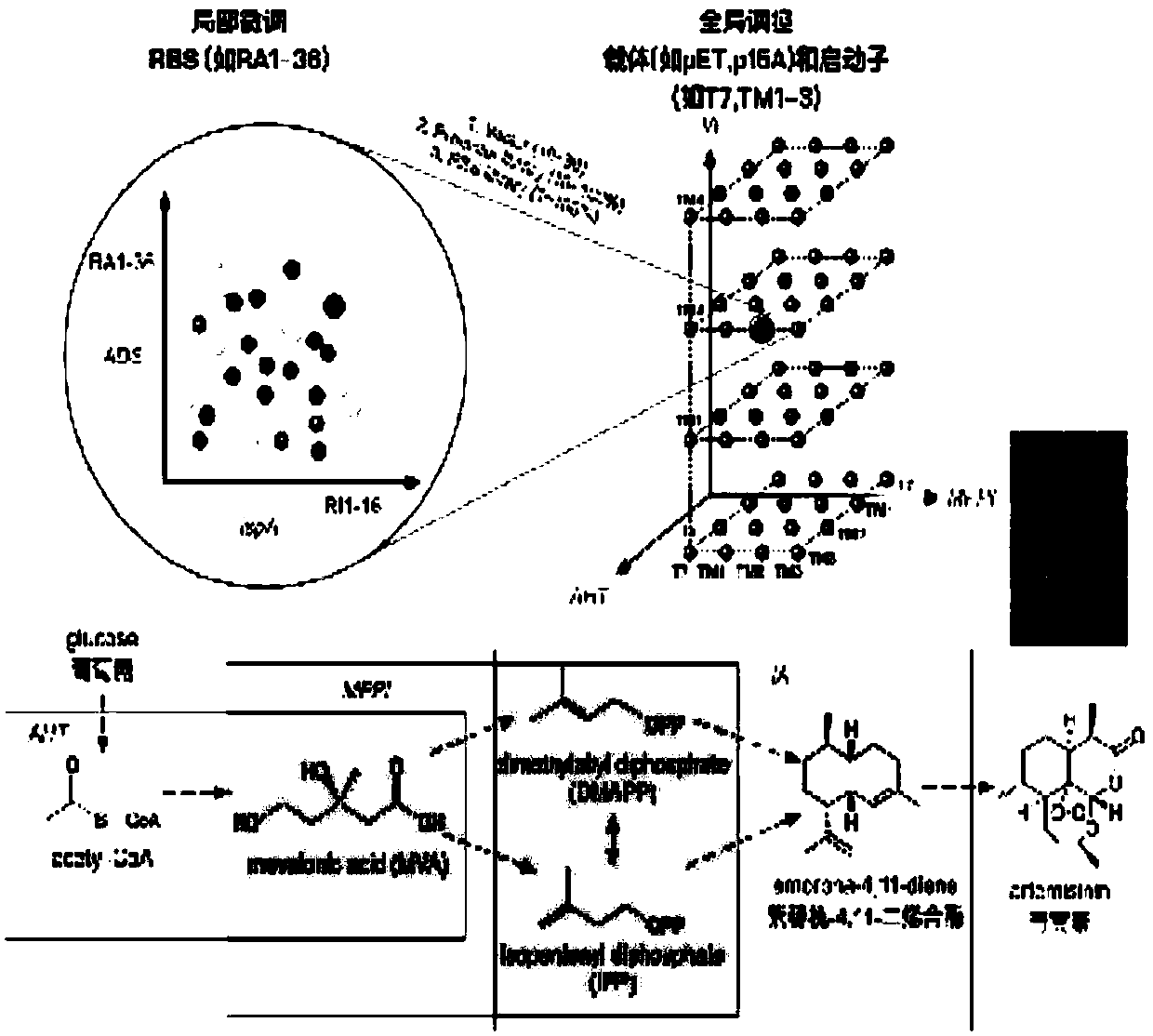
[0025] The biosynthetic pathway of this embodiment is as figure 1 As shown, the construction methods of engineered bacteria include global optimization and local optimization, specifically by using predefined regulatory elements (regulatory elements refer to vectors, promoters and 5'untranslated regions) to finely assemble the bacterial library in a high-dimensional, full-combination manner, And combined with analytical chemistry (gas mass spectrometer) to screen the highest yield of microbial strains.
[0026] The flow chart of manufacturing engineered bacteria producing high-value natural products, figure 1 Shown:
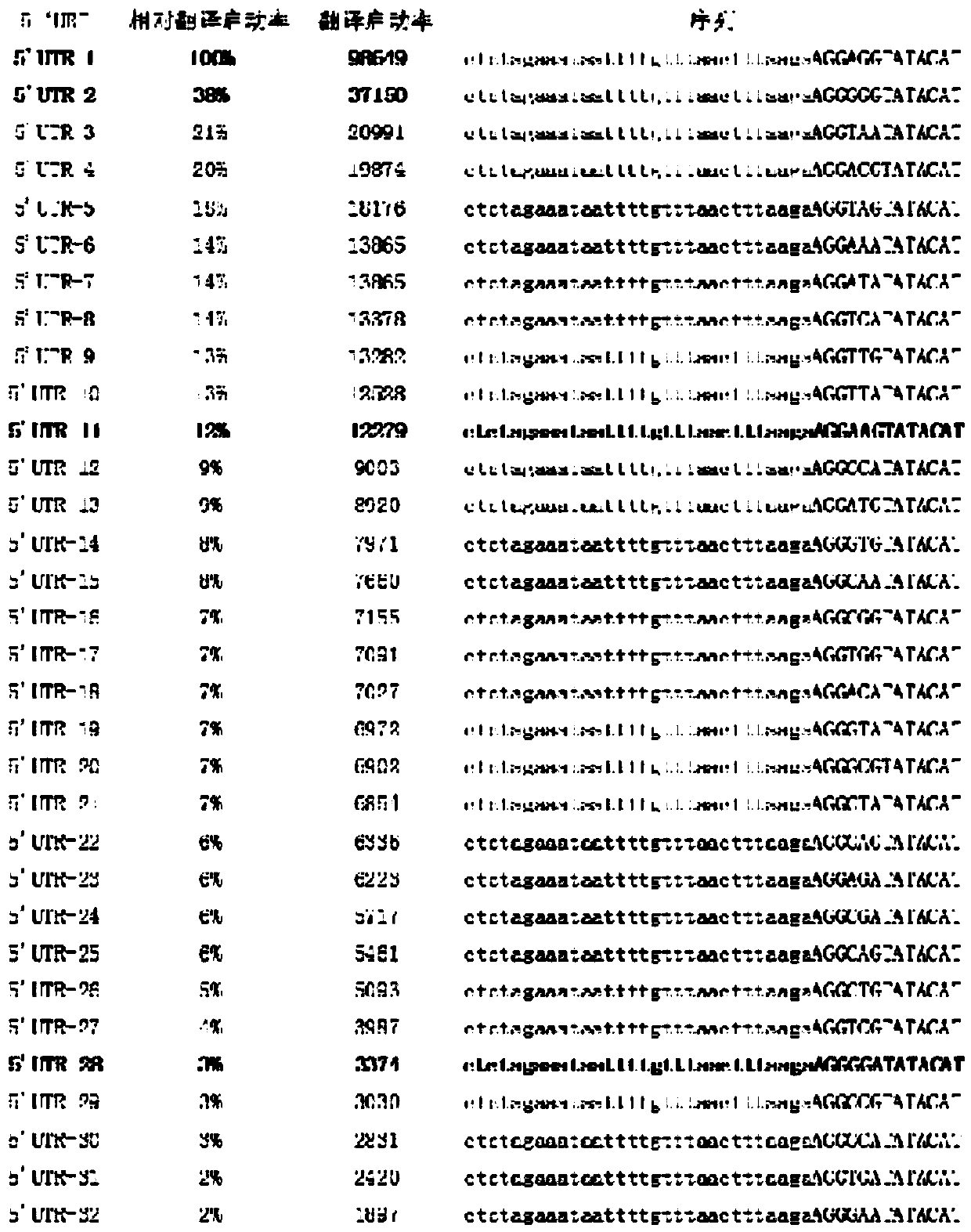
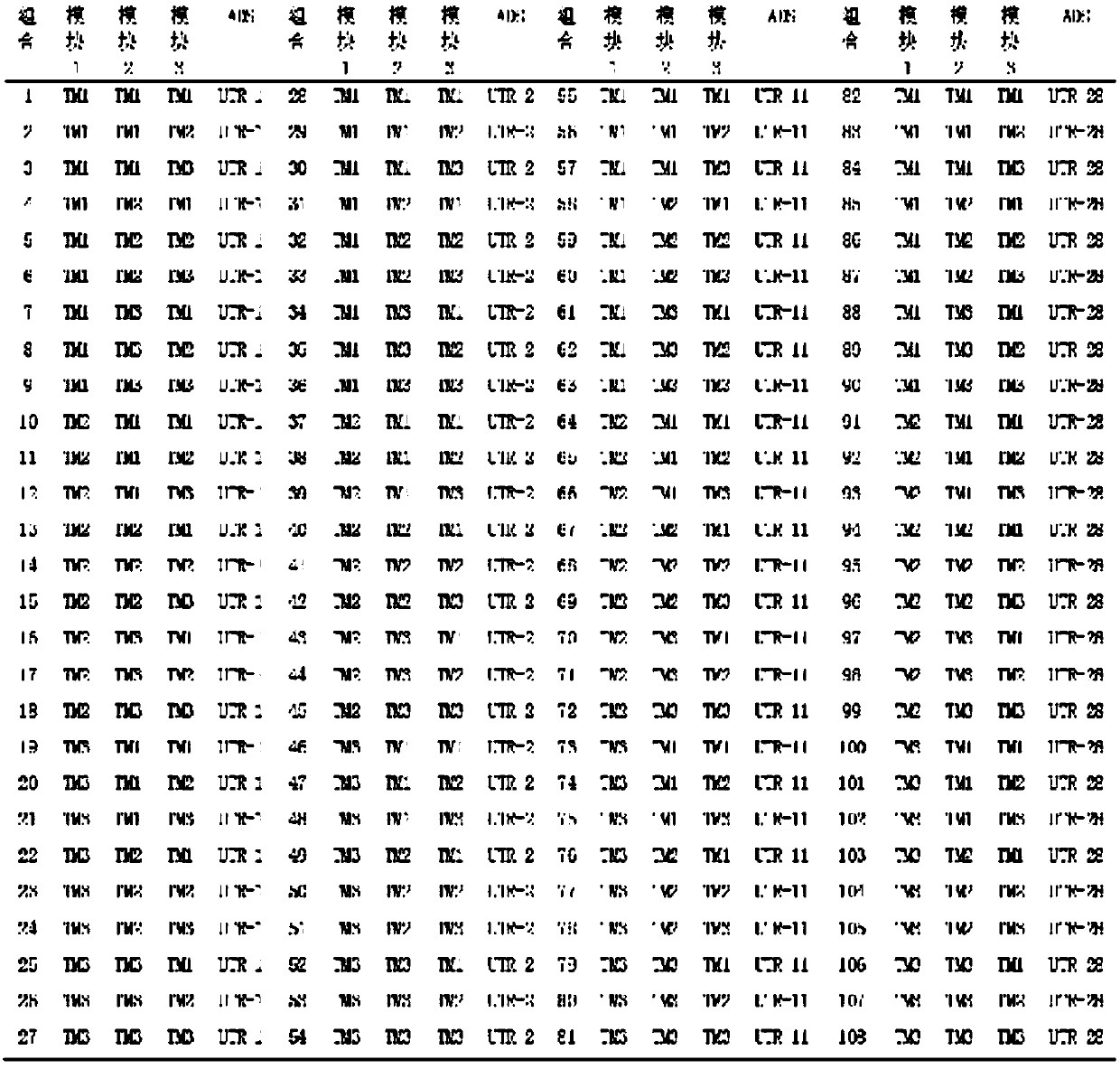
[0027] (1) First, establish and characterize the promoter and RBS library through experimental or mathematical methods. The promoter library (T7, TM1-255) was established through experiments, and the specific details can be found in the patent application 2017110721556. The 5’ untranslated region library was established and char...
Embodiment 2
[0033] Example 2: Optimization of basic medium
[0034] Step 1) Prepare 5L of chemically determined medium, including 10g / L glucose, 2g / L(NH 4 ) 2 SO 4 , 4.2g / LKH 2 PO 4 And 11.24g / LK 2 HPO 4 , 1.7g / L citric acid, 0.5g / L MgSO 4 And 10mL / L trace element solution. Among them, the trace element solution contains 0.25g / L CoCl 2 ·6H 2 O, 1.5g / L MnSO 4 ·4H 2 O, 0.15g / L CuSO 4 ·2H 2 O, 0.3g / LH 3 BO 3 , 0.25g / L Na 2 MoO 4 ·2H 2 O,0.8g / L Zn(CH 3 COO) 2 , 5g / L iron (III) citrate and 0.84g / L EDTA, the pH of the trace element solution is 8.0.
[0035] Step 2) Sterilize the culture medium at 121°C for 15-20 minutes. (Note, the glucose solution should be sterilized separately and then mixed with other ingredients).
[0036] Step 3) Next, the strain 323-2 is pre-cultured in the chemically determined medium of Step 2, and cultured at 37°C for about 14 hours to prepare a bacterial solution.
[0037] Step 4) Inoculate 1% of strain 323-2 into 25 mL of the medium of step 2, and compare the effects of di...
Embodiment 3
[0040] Example 3: Optimization of sampling medium for fed-batch fermentation
[0041] Step 1) Prepare 5L of chemically determined medium, including 10g / L glucose, 2g / L(NH 4 ) 2 SO 4 , 4.2g / LKH 2 PO 4 And 11.24g / L K 2 HPO 4 , 1.7g / L citric acid, 0.5g / L MgSO 4 And 10mL / L trace element solution. Among them, the trace element solution contains 0.25g / L CoCl 2 ·6H 2 O, 1.5g / L MnSO 4 ·4H 2 O, 0.15g / L CuSO 4 ·2H 2 O, 0.3g / LH 3 BO 3 , 0.25g / L Na 2 MoO 4 ·2H 2 O,0.8g / L Zn(CH 3 COO) 2 , 5g / L iron (III) citrate and 0.84g / L EDTA, the pH of the trace element solution is 8.0.
[0042] Step 2) Sterilize the culture medium at 121°C for 15-20 minutes. (Note, the glucose solution should be sterilized separately and then mixed with other ingredients).
[0043] Step 3) Next, the strain 323-2 is pre-cultured in the medium with determined chemical composition in Step 2, and cultured at 37°C for about 14 hours to prepare a bacterial solution.
[0044] Step 4) Inoculate 2% strain 323-2 into the fermentor, us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com