Synthesis method and application of 2-hydroxy-N-(4'-chlorobiphenyl-2-yl)nicotinamide
A synthetic method, the technology of chlorinated biphenyls, applied in the field of pesticide fungicides, can solve the problem of low nicotinamide yield and achieve the effect of simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
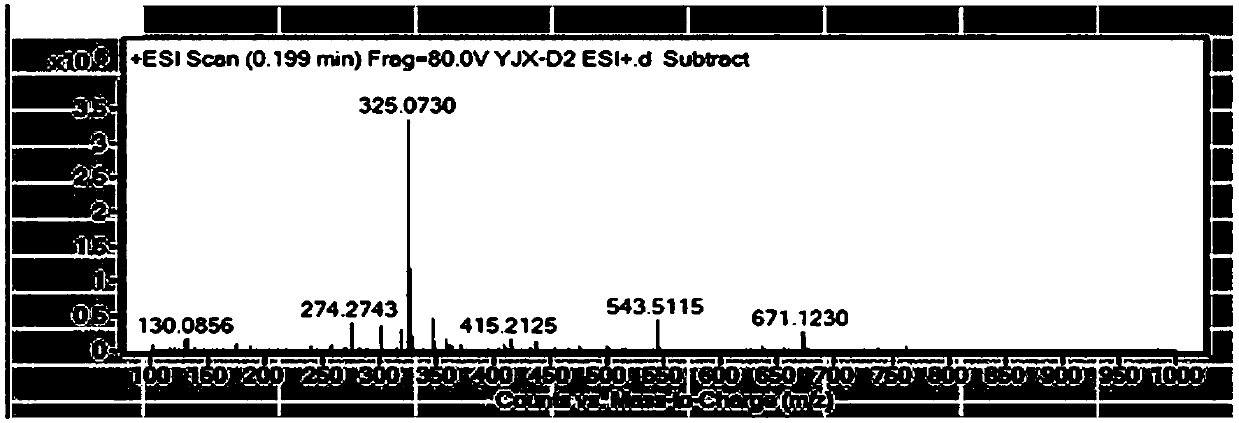
[0049] Example 1 The synthetic method of 2-hydroxyl-N-(4'-chlorobiphenyl-2-yl) nicotinamide
[0050] Using 2-chloronicotinic acid and 4'-chloro-2-aminobiphenyl as raw materials, react with catalyst 3-nitrophenylboronic acid in toluene solution at 120°C to generate 2-hydroxy-N-(4'-chlorobiphenyl- 2-yl) nicotinamide, the specific synthetic route is as follows:
[0051]
[0052] Step 1): Measure 1.56kg (ie 10mol) of 2-chloronicotinic acid, 1.02kg (ie 5mol) of 4'-chloro-2-aminobiphenyl, and 0.42kg (ie 2.5mol) of 3-nitrobenzene Boric acid, spare;
[0053] Measure 30L of toluene, add 2-chloronicotinic acid to toluene, stir to dissolve at room temperature, add 4'-chloro-2-aminobiphenyl, stir and mix, then add 3-nitrophenylboronic acid, heat to 120°C, And after keeping at 120°C for 10 hours, the solution B was prepared, and the temperature of the solution B was naturally cooled to room temperature;
[0054] Step 2): Add 30L of saturated aqueous sodium bicarbonate solution to solut...
Embodiment 2-9
[0060] Example 2-9 The synthetic method of 2-hydroxyl-N-(4'-chlorobiphenyl-2-yl)nicotinamide
[0061] Examples 2-9 are respectively the synthesis method of 2-hydroxy-N-(4'-chlorobiphenyl-2-yl) nicotinamide, the synthesis method is the same as that of Example 1, the difference is that the above-mentioned 2-hydroxy- The various process parameters in the N-(4'-chlorobiphenyl-2-yl) nicotinamide process are different, see Table 1 for details:
[0062] List of various process parameters in Table 1 Embodiment 2-9
[0063]
[0064] The following is the refining method of 2-hydroxyl-N-(4'-chlorobiphenyl-2-yl) nicotinamide synthesized in Example 2-9, the refining method is the same as in Example 1, the difference is that the above-mentioned 2 -Hydroxy-N-(4'-chlorobiphenyl-2-yl) nicotinamide process conditions are different, see Table 2 for details:
[0065] List of various refining process conditions in table 2 embodiment 2-9
[0066]
Embodiment 10
[0067] Example 10 Application of 2-hydroxyl-N-(4'-chlorobiphenyl-2-yl)nicotinamide
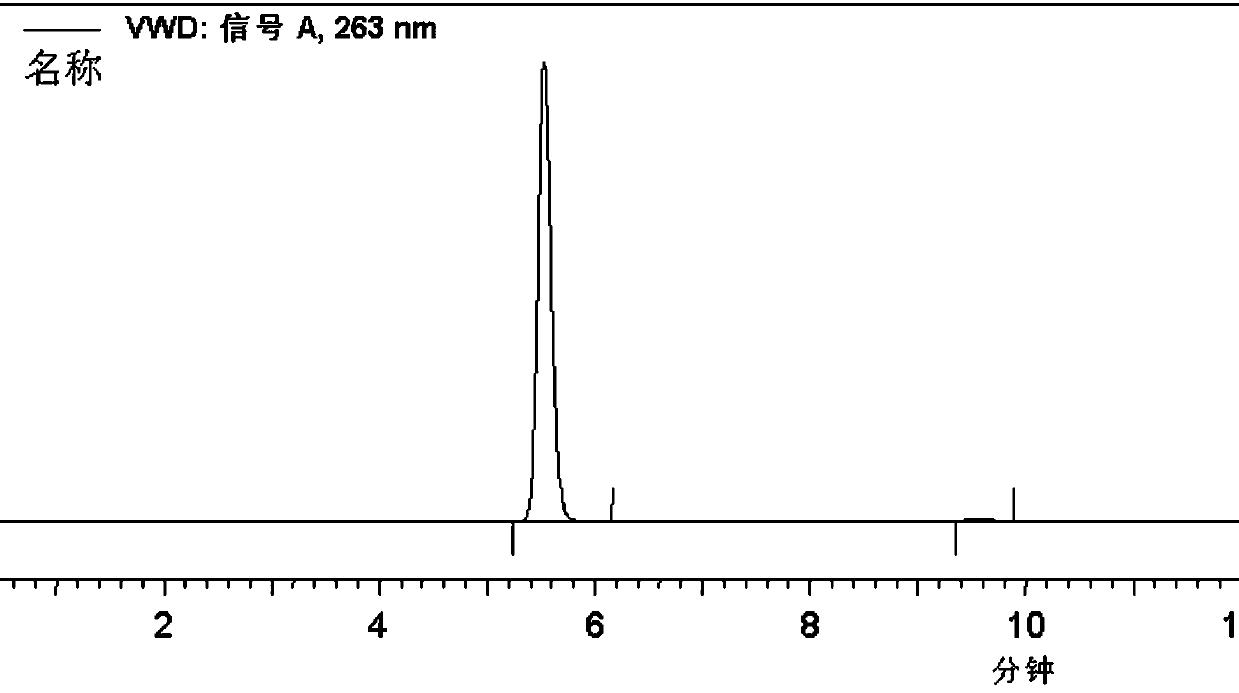
[0068] 2-Hydroxy-N-(4'-chlorobiphenyl-2-yl)nicotinamide fine product prepared in Example 1, as a standard reference substance, by comparing 2-hydroxy-N-(4'-chlorobiphenyl-2 - base) liquid phase diagram of nicotinamide and boscalid to detect the presence of 2-hydroxy-N-(4'-chlorobiphenyl-2-yl) nicotinamide and The amount of the content can realize the monitoring of the synthesis process of boscalid and the detection of the quality of boscalid products.
[0069] Monitoring the synthesis of boscalid:
[0070] Producing boscalid according to the prior art, p-chlorobromobenzene makes p-chlorophenylboronic acid through Grignard reaction, then with o-chloronitrobenzene in catalyst MS-Pd, anhydrous potassium carbonate and tetrabutylammonium bromide ( In the presence of TBAB), a Suzuki reaction occurs at 120°C in DMF to generate 2-(4-chlorophenyl)nitrobenzene; then 2-(4-chlorophenyl)aniline is obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com