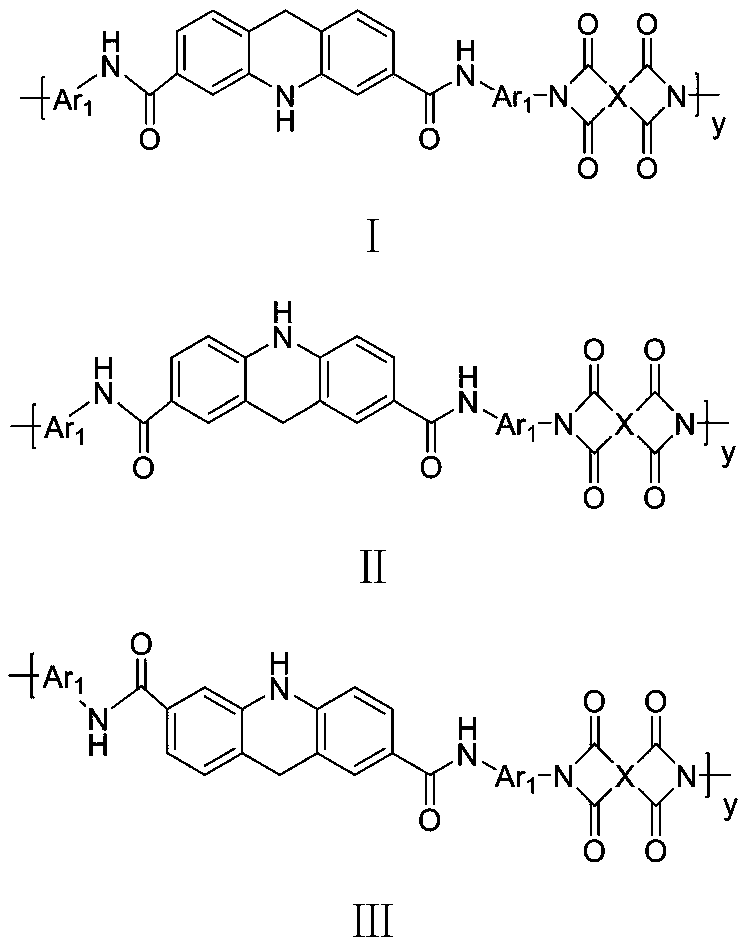
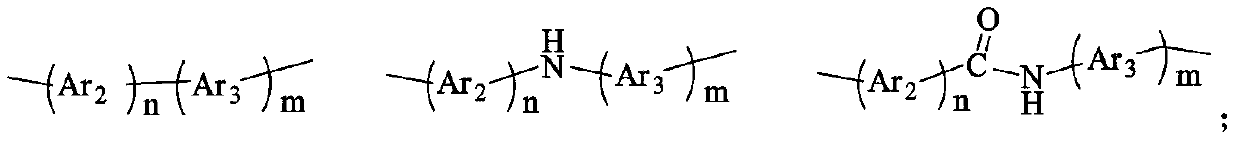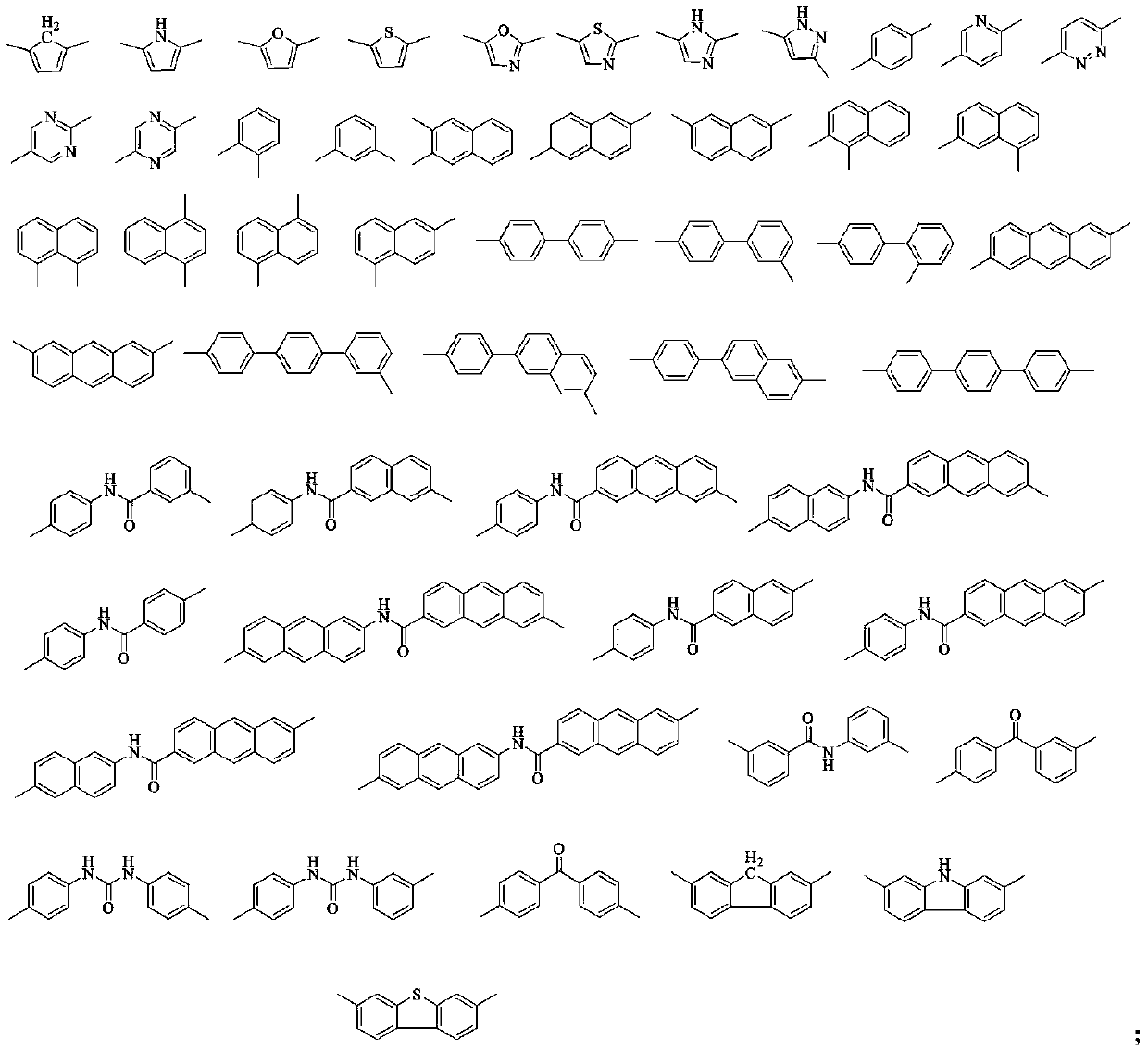High-barrier polyimide containing 9,10-dihydroacridine structure, and preparation method and application thereof
A technology of dihydroacridine and polyimide, which is applied in the field of material science to achieve the effect of high planarity, promotion of close packing, and regular arrangement of molecular chains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This example provides
[0049] Synthesis of N2,N7-bis(4-aminophenyl)-9,10-dihydroacridine-2,7-dicarboxamide:
[0050]
[0051] S1. Synthesis of intermediate 9,10-dihydroacridine-2,7-dicarbonitrile:
[0052] Add 3.39g (0.01mol) of 2,7-dibromo-9,10-dihydroacridine, 4.478g (0.05mol) of cuprous cyanide, and 50ml of dry NMP into a 500ml three-necked flask, reflux at 140°C for 24h, and then add H 2 O (180 mL), HCl (60 mL) and FeCl3 (4.19 g, 25.8 mmol) were poured into the reaction solution and stirred for 1 h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, and the obtained solid was redissolved in dichloromethane and washed with water , the solvent was removed under reduced pressure to give the crude product as a brown solid which was triturated with methanol to give the intermediate. The intermediate structure is as follows:
[0053]
[0054] S2. Synthesis of intermediate 9,10-dihydroacridine-2,7-dicarboxylic acid:
[005...
Embodiment 2
[0067] This example provides
[0068] Synthesis of N2,N6-bis(5-aminothiophen-2-yl)-9,10-dihydroacridine-2,6-dicarboxamide:
[0069]
[0070] S1. Synthesis of intermediate 9,10-dihydroacridine-2,6-dicarbonitrile:
[0071] Add 3.39g (0.01mol) of 2,6-dibromo-9,10-dihydroacridine, 2.7624g (0.05mol) of cyanocopper, and 50ml of dry NMP into a 500ml three-neck flask, reflux at 140°C for 24h, then add water (180mL), HCl (60mL) and FeCl3 (4.19g, 25.8mmol) were poured into the reaction solution and stirred for 1h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, the resulting solid was re-dissolved in dichloromethane and washed with water, and the solvent was removed under reduced pressure The crude product was obtained as a brown solid which was triturated with methanol to give the intermediate. The intermediate structure is as follows:
[0072]
[0073] S2. Synthesis of intermediate 9,10-dihydroacridine-2,6-dicarboxylic acid:
[0074...
Embodiment 3
[0087] This example provides a synthetic
[0088] N3,N6-bis(7-aminodibenzo[b,d]furan-3-yl)-9,10-dihydroacridine-3,6-dicarboxamide:
[0089]
[0090] S1. Synthesis of intermediate 9,10-dihydroacridine-3,6-dicarbonitrile:
[0091] Add 3.39g (0.01mol) of 3,6-dibromo-9,10-dihydroacridine, 2.7624g (0.05mol) of cyanocopper, and 50ml of dry NMP into a 500ml three-neck flask, reflux at 140°C for 24h, then add water (180mL), HCl (60mL) and FeCl3 (4.19g, 25.8mmol) were poured into the reaction solution and stirred for 1h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, the resulting solid was re-dissolved in dichloromethane and washed with water, and the solvent was removed under reduced pressure The crude product was obtained as a brown solid which was triturated with methanol to give the intermediate. The intermediate structure is as follows:
[0092]
[0093] S2. Synthesis of intermediate 9,10-dihydroacridine-3,6-dicarboxylic acid: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com