Device and method for synthesizing ammonia through electro-catalytic nitrogen reduction by replacing Nafion membrane with salt bridge
A technology for catalyzing nitrogen and synthesizing ammonia, which is applied in the electrolysis process, electrolysis components, cells, etc., can solve the problems of inaccurate evaluation of catalyst performance and ammonia measurement error, and achieve long-term stable operation, elimination of diffusion, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
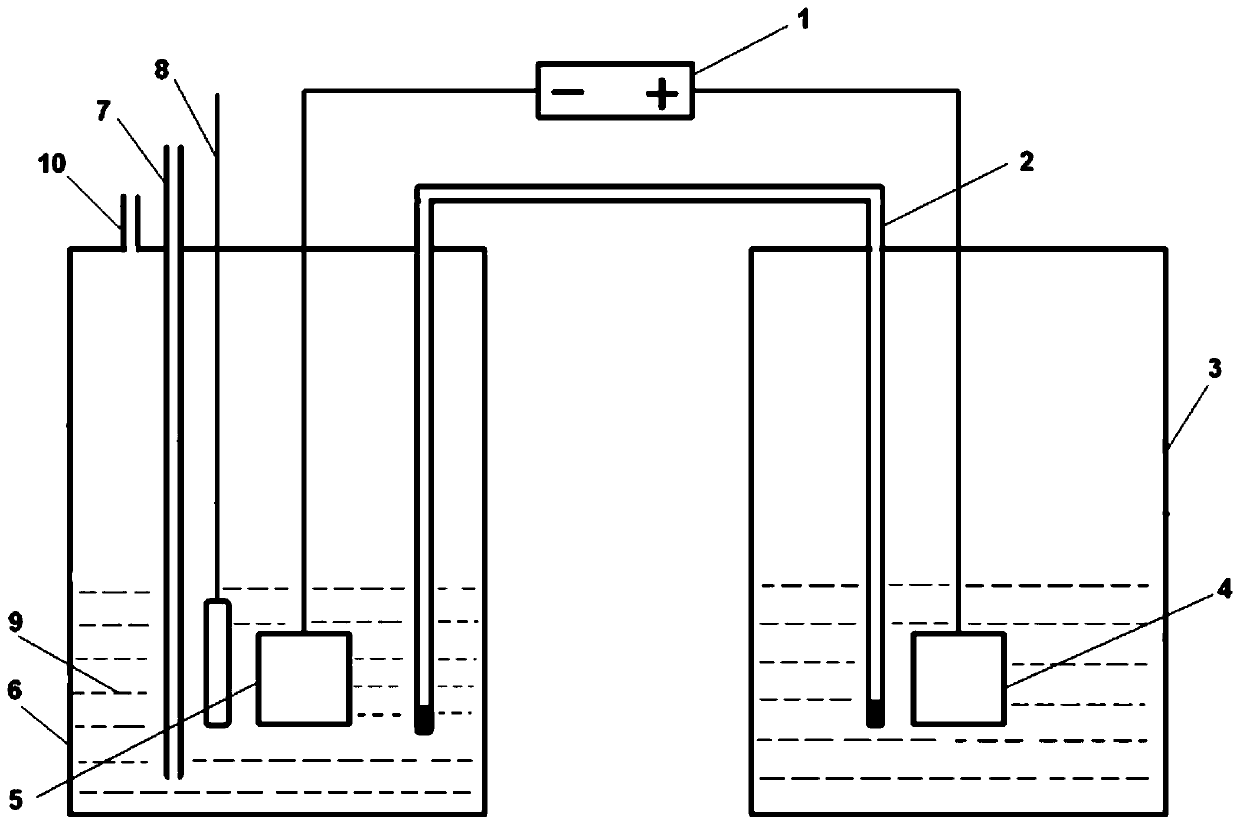
[0024] Such as figure 1 As shown, an electrocatalytic nitrogen reduction ammonia synthesis device that replaces the Nafion membrane with a salt bridge includes an anode cell 3 and a cathode cell 6 filled with an electrolyte 9, and an anode plate 4 is suspended inside the anode cell 3 and immersed in the electrolyte , the anode plate 4 is connected to the positive pole of the power supply 1 through a wire, the cathode plate 5 and the reference electrode 8 are suspended inside the cathode pool 6 and immersed in the electrolyte, and the inside of the cathode pool 6 is provided with a nitrogen gas inlet pipeline 7 and immersed in the electrolyte , the top of the cathode pool 6 is also provided with a gas outlet 10, the cathode plate 5 is connected to the negative pole of the power supply 1 through a wire, and the anode pool 3 and the cathode pool 6 filled with electrolyte are connected through a salt bridge 2, and the salt bridge 2 is U-shaped The round or square pipes are made of...
Embodiment 2
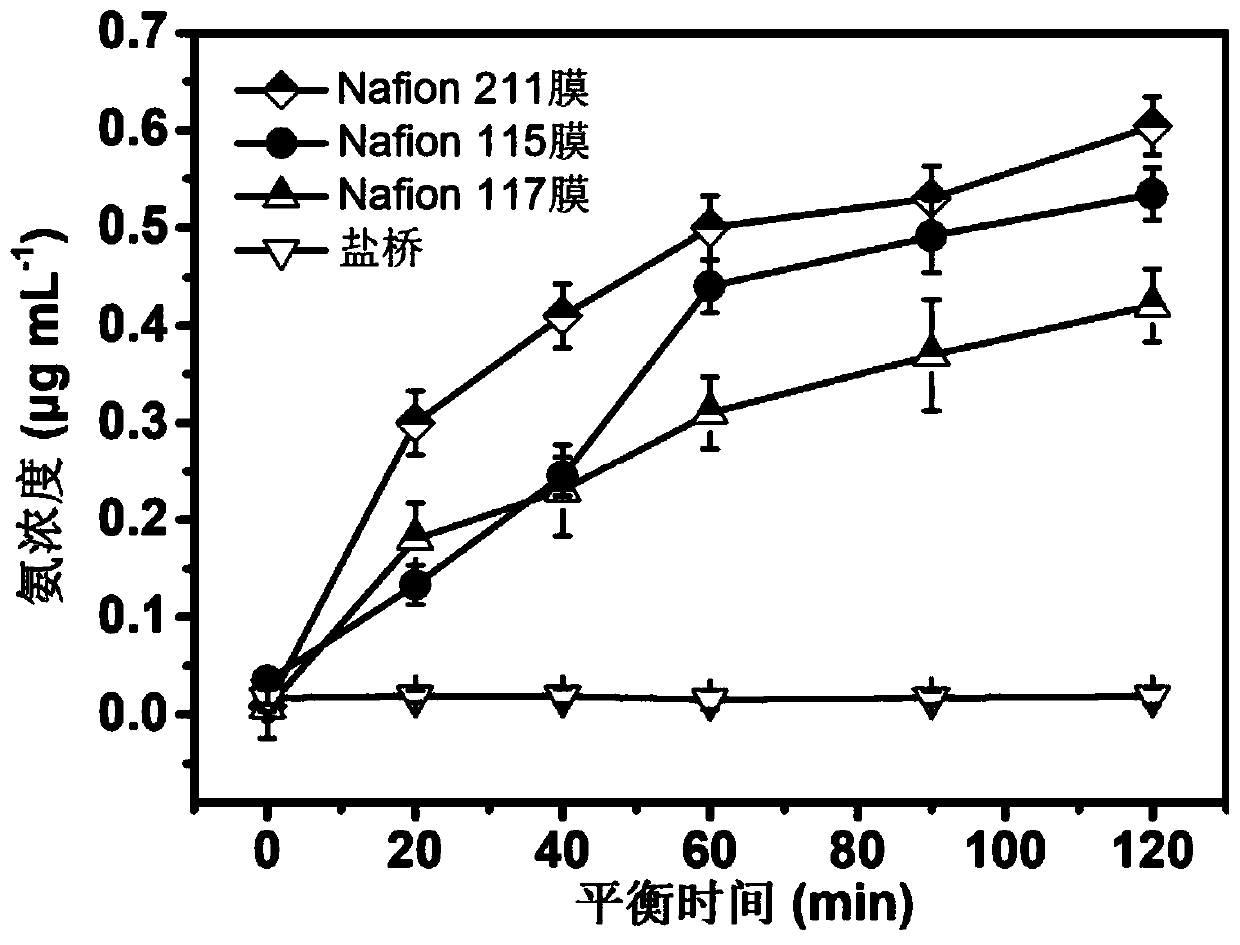
[0026] The present invention was compared to ammonia diffusion in conventional devices equipped with Nafion membranes (Nafion 115, Nafion 117 and Nafion 211). The specific operation is as follows: 30 mL of ammonia-containing KOH electrolyte (the ammonia concentration is 2 μg mL -1 ), while 30mL of ammonia-free KOH electrolyte is housed in the anode cell. The material of the salt bridge is selected from glass, and the inside is filled with saturated potassium chloride solution. The two ports of the salt bridge are sealed with agar-containing absorbent cotton and immersed in the electrolyte. The change of the ammonia concentration of the electrolyte in the anode pool of the two devices with the equilibrium time was measured respectively by Nessler's reagent spectrophotometry, as figure 2 As shown, in the traditional device equipped with Nafion membrane, the ammonia concentration of the electrolyte in the anode cell showed an upward trend with the increase of the equilibrium ti...
Embodiment 3
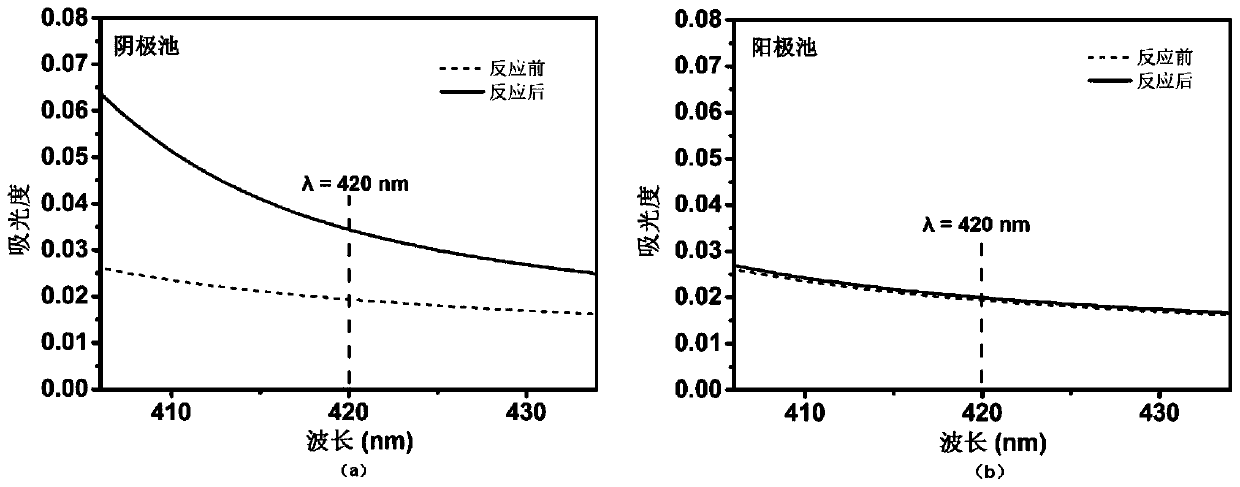
[0028] Inject 30mL (0.1M KOH) electrolyte into the cathode cell and the anode cell respectively to load RuO 2 1×1cm 2 The carbon paper (loading capacity 0.1mg) is the cathode plate, the platinum sheet is the anode plate, the Hg / HgO (1M KOH) electrode is the reference electrode, the material of the salt bridge is selected from plexiglass, and the interior is filled with saturated sodium chloride solution, salt The two ports of the bridge are sealed with glass sand cores and immersed in the electrolyte. Open the inlet of the nitrogen gas inlet line, at 20mL min -1 After passing high-purity nitrogen (99.999% purity) at a flow rate for 30 minutes, turn on the power switch, set the required voltage to carry out the nitrogen reduction process at the potential of -0.15V vs. reversible hydrogen electrode (RHE), and the electrolysis time is 2 hours. Nessler's reagent spectrophotometry was used to detect the change of absorbance of the electrolyte in the cathode cell and the anode cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com