Iridium complex constructed based on 8-hydroxyquinoline derivative and 2-phenylpyridine iridium dimer and synthesis method and application thereof
A synthesis method and complex technology, applied in the field of medicine, can solve problems such as the synthesis method and application of iridium complexes, obvious side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of complex Ir1
[0037] In a 100.0mL round bottom flask, add 2.0mmol of chloroquinaldol (H-QL1) and 1.0mmol of 2-phenylpyridine iridium dimer, and then add 16.5mL of organic solvent (by 15.0mL of ethanol and 1.5mL Composition of water), stirred to dissolve, reacted at 70°C until complete (about 12h), stopped the reaction, cooled to room temperature, reddish-brown crystals were precipitated, collected and dried to obtain a reddish-brown solid product. Yield 90.21%.
[0038] The product obtained in this embodiment is characterized:
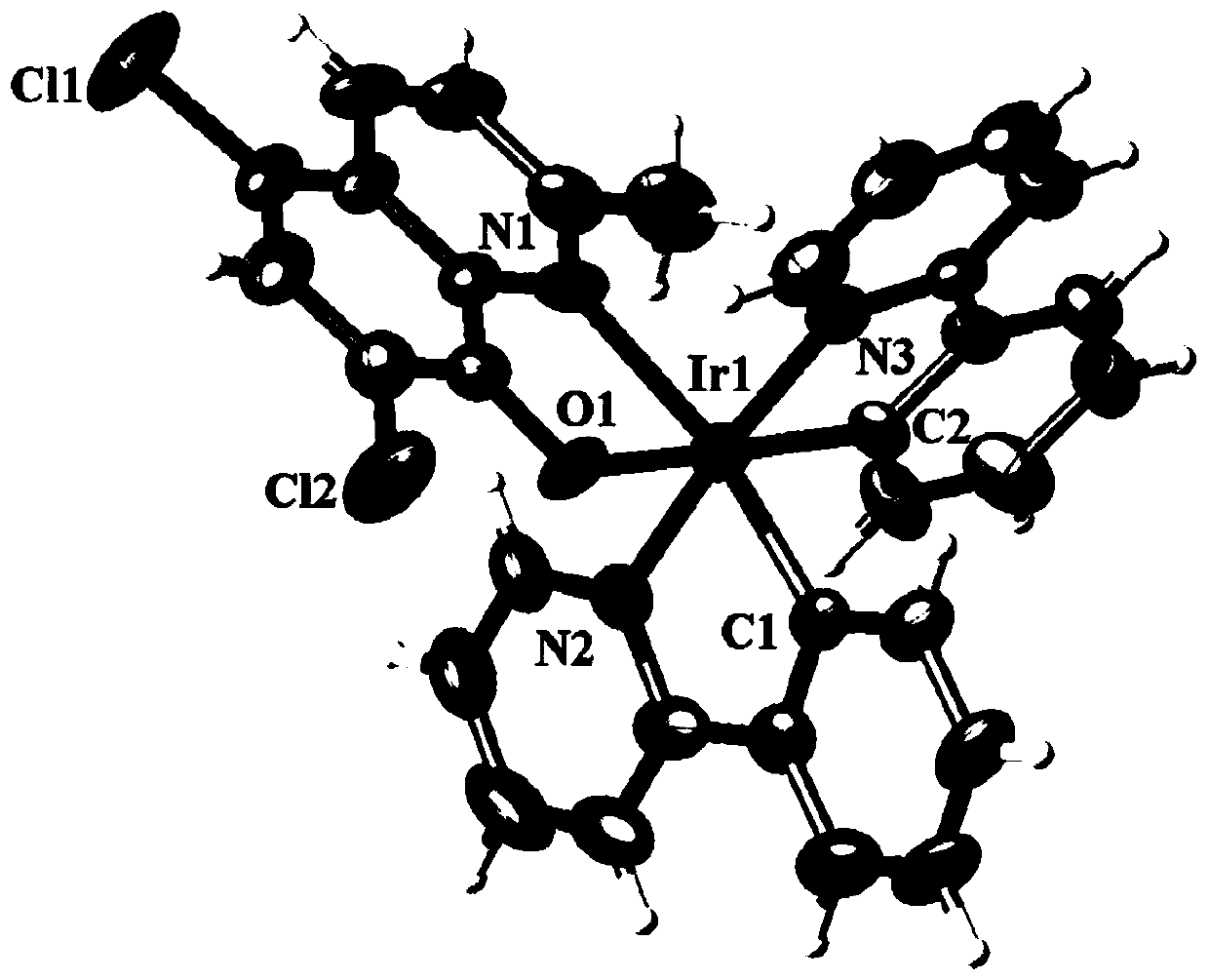
[0039] (1) X-ray single crystal diffraction
[0040] The reddish-brown crystal with perfect surface structure was measured by single crystal diffraction to determine its crystal structure. The obtained crystallographic and structural correction data are shown in Table 1 below, and the data of some bond lengths and angles are shown in Table 2 and Table 3 below. The crystal structure of reddish-brown crystals is as ...
Embodiment 2
[0059] Embodiment 2: the synthesis of complex Ir1
[0060] Example 1 was repeated, except that the organic solvent was changed to ethanol, and the amount used remained unchanged; the reaction was changed to 100° C. (the reaction was completed for about 6 hours).
[0061] The result is reddish-brown crystals. Yield 85.31%.
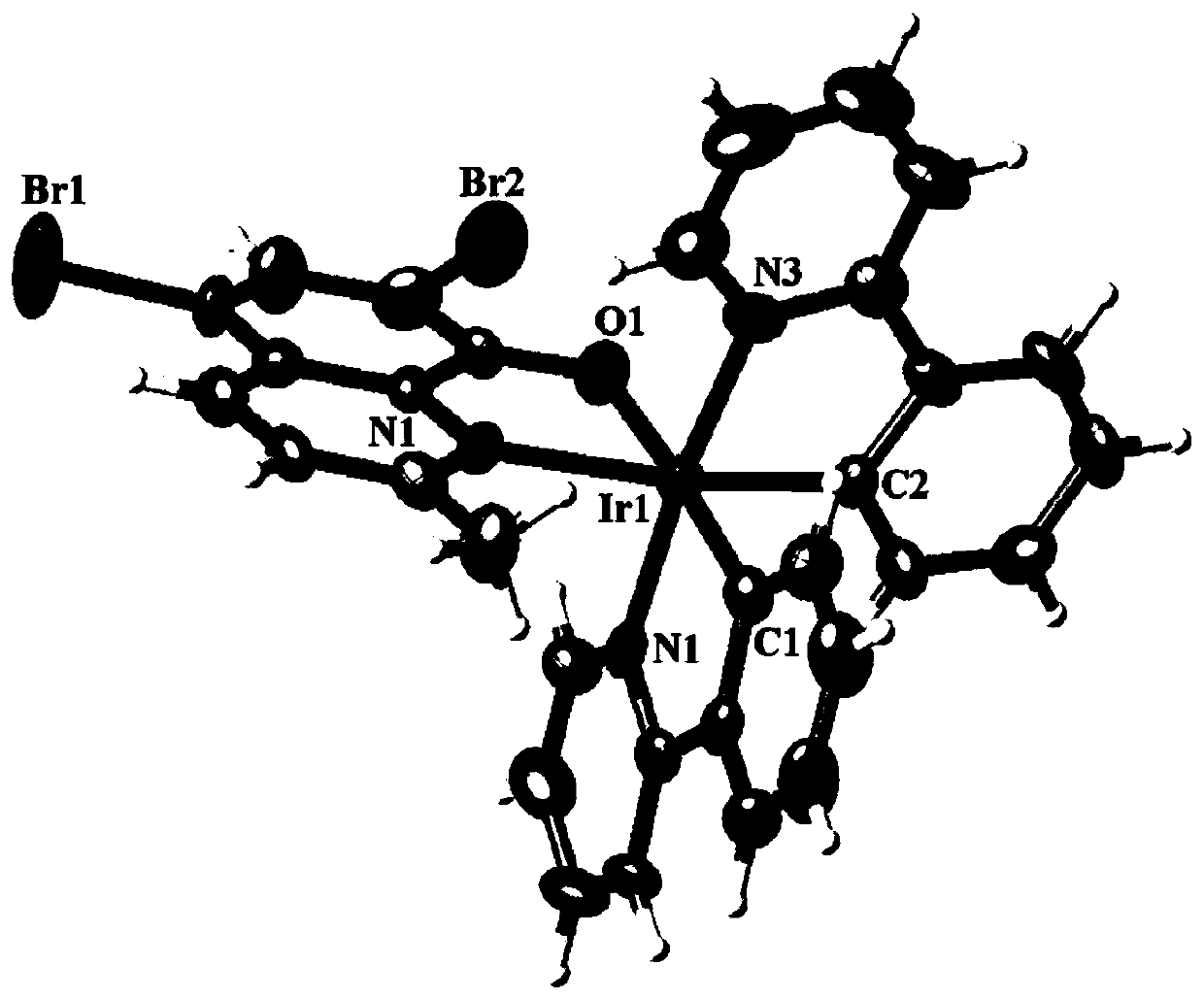
[0062] Single crystal diffraction analysis, elemental analysis, infrared analysis and mass spectrometry analysis were performed on the product obtained in this example, and it was determined that the obtained reddish-brown crystal was the target complex Ir1.
Embodiment 3
[0063] Embodiment 3: the synthesis of complex Ir2
[0064] In a 100.0mL round bottom flask, add 2.0mmol of 5,7-dibromo-2-methyl-8-hydroxyquinoline (H-QL2) and 1.0mmol of 2-phenylpyridine iridium dimer respectively, and then Add 16.5mL of organic solvent (consisting of 3.5mL of ethanol and 10.5mL of chloroform), stir to dissolve, carry out the reaction at 65°C until complete (about 27h), stop the reaction, cool to room temperature, and reddish-brown crystals precipitate out. The crystals were collected and dried to obtain a reddish-brown solid product. Yield 80.46%.
[0065] The product obtained in this embodiment is characterized:
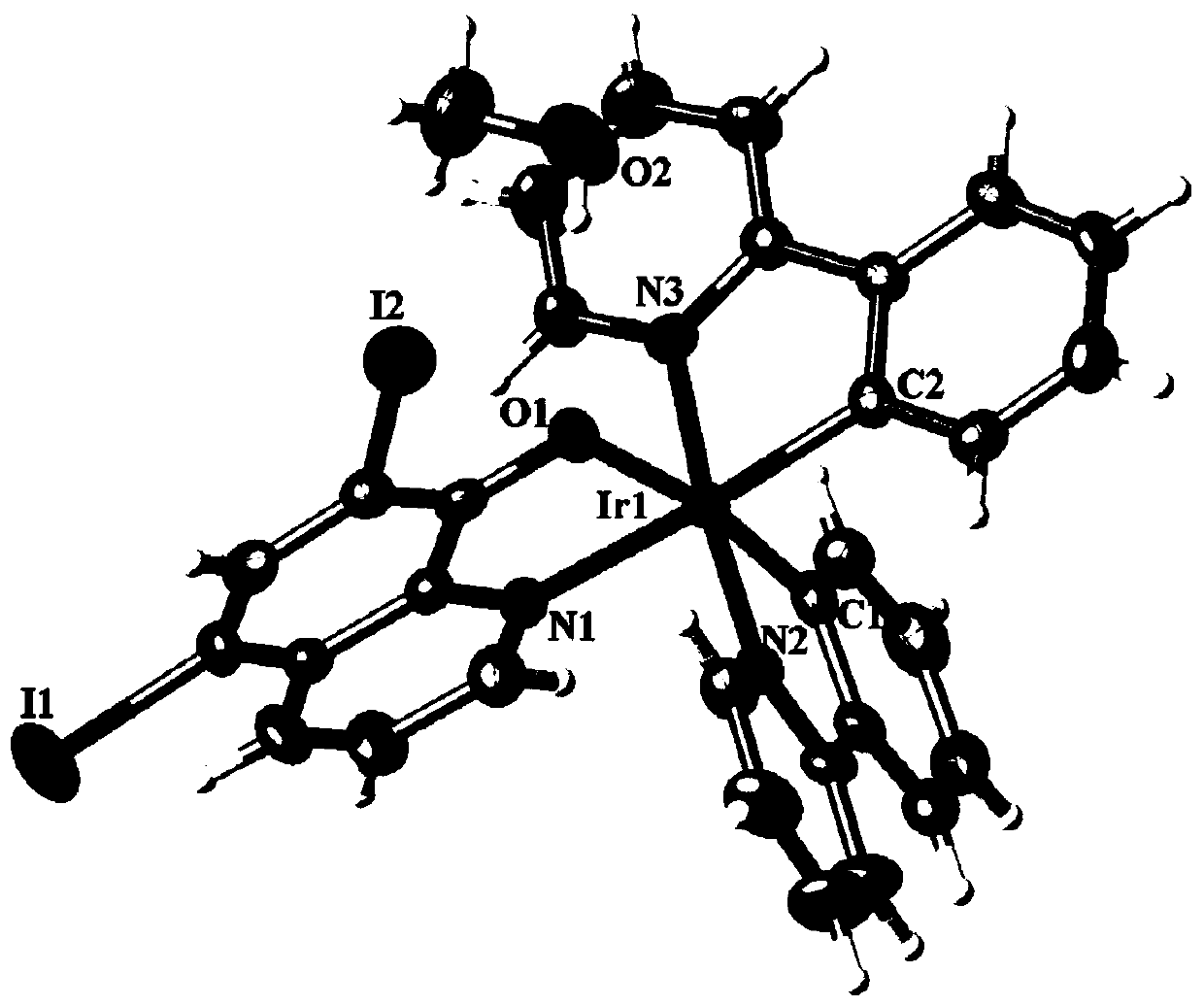
[0066] (1) X-ray single crystal diffraction
[0067] The reddish-brown crystal with perfect surface structure was measured by single crystal diffraction to determine its crystal structure. The obtained crystallographic and structural correction data are shown in the above Table 1, and some bond length and bond angle data are shown in the followi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com