Ultrafine arsenic removal adsorbent and preparation method thereof, and arsenic removal method
A technology of adsorbent and surfactant, which is applied in the field of ultrafine arsenic removal adsorbent and its preparation, can solve the problems of difficult arsenic removal, low arsenic content, etc., and achieve increased arsenic removal effect, high specific surface area, and reaction The effect of increased active sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of an ultrafine arsenic-removing adsorbent, comprising the following steps:
[0046] 1) take by weighing the calcium chloride of 2.22g, the sodium metaaluminate of 0.55g, 0.47g sodium sulfate (Ca 2+ :Al 3+ :SO 4 2- The molar ratio is 6:2:1), the three raw materials were dissolved in 200ml deionized water respectively, and then the obtained three solutions were mixed, and 5% of the total mass of the raw materials (calcium chloride, sodium metaaluminate and sodium sulfate) was added EDTA disodium, using NaOH solution to adjust the pH value of the mixed solution to 11.5;
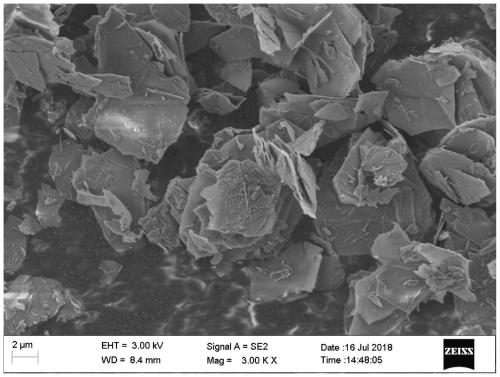
[0047] 2) Seal the solution prepared in step 1), heat and adjust the temperature to 90°C, and stir magnetically under this condition for 12 hours, then filter the synthesized suspension solution and wash the precipitate until neutral, vacuum-dry at 50°C for 24 hours, and store in a sealed container. Standby, denoted as AFm-1, the scanning electron microscope appearance is as foll...
Embodiment 2
[0049] A preparation method of an ultrafine arsenic-removing adsorbent, comprising the following steps:
[0050] 1) Weigh 3.33g of calcium chloride, 0.83g of sodium metaaluminate, and 0.71g of sodium sulfate, dissolve the three kinds of raw materials in 200ml of deionized water respectively, then mix the obtained three kinds of solutions, add the total amount of raw materials 5 % of disodium edetate, using NaOH solution to adjust the pH value of the mixed solution to 11.5;
[0051] 2) Seal the solution prepared in step 1), seal it, heat and adjust the temperature to 90°C, and stir it magnetically for 12 hours under this condition, then filter the synthesized suspension solution and wash the precipitate to neutrality, vacuum-dry it at 50°C for 24 hours, and seal it Storage, standby, recorded as AFm-2, scanning electron microscope appearance as figure 2 shown.
Embodiment 3
[0053] A preparation method of an ultrafine arsenic-removing adsorbent, comprising the following steps:
[0054] 1) Weigh 3.33g of calcium chloride, 0.825g of sodium metaaluminate, and 0.71g of sodium sulfate, dissolve the three kinds of raw materials in 200ml of deionized water respectively, then mix the obtained three kinds of solutions, add the total amount of raw materials 5 % of disodium edetate, use the configured NaOH solution to adjust the pH value of the mixed solution to 11.5;
[0055] 2) Seal the solution prepared in step 1), seal it, heat it to adjust the temperature to 90°C, and stir it magnetically for 24 hours under this condition, then filter and wash the synthesized suspension solution until it is neutral, vacuum-dry it at 50°C for 24 hours, and store it sealed , spare, denoted as AFm-3, scanning electron microscope appearance as image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com