Targeting vector, construction method for transgenic mouse capable of regulating and removing monocyte-derived DC via diphtheria toxin, and application of targeting vector
A technology for transgenic mice and targeting vectors, applied in biochemical equipment and methods, other methods of inserting foreign genetic materials, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
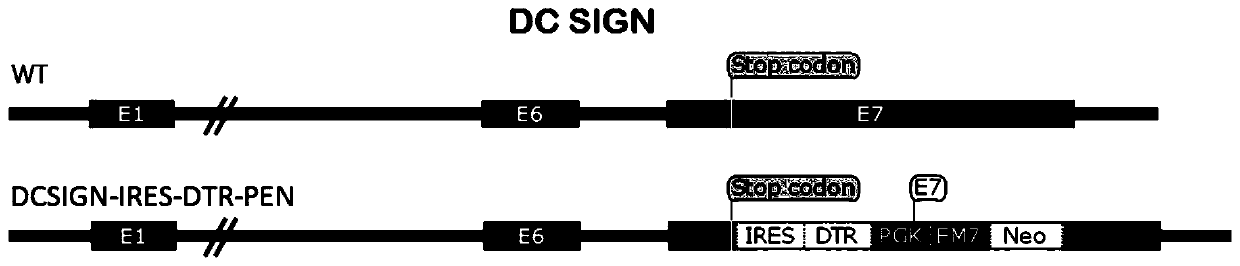
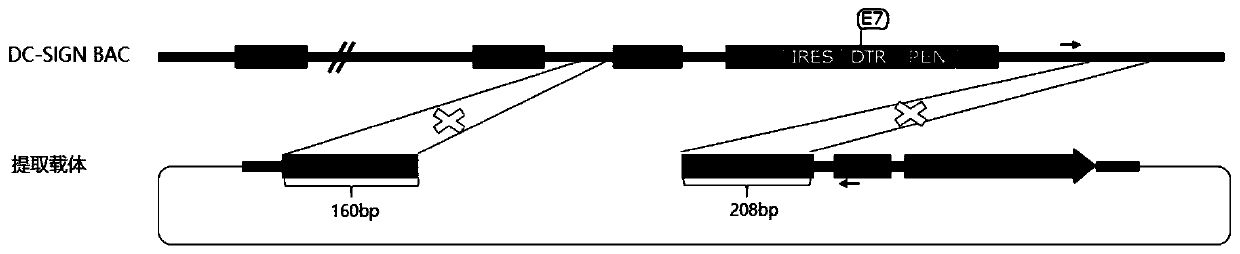
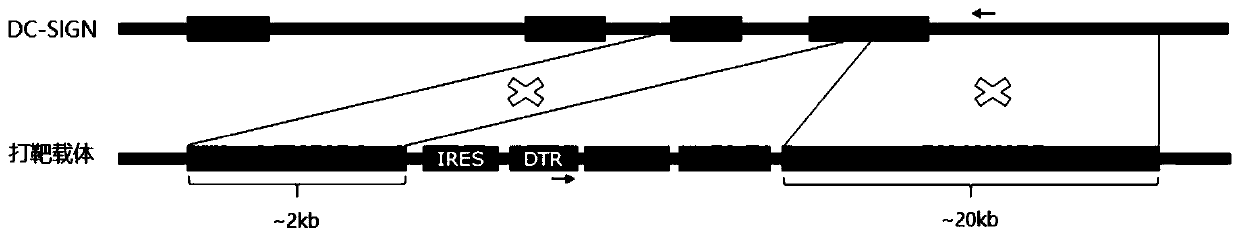
[0053] Example 1: Construction of a targeting vector with a long homology arm that targets the integration of the exogenous gene IRES-DTR into exon 7 of DC-SIGN.
[0054] The mouse DC-SIGN genome sequence was retrieved and downloaded from the genome.ucsc.edu website, combined with the mouse DC-SIGN (Genbank No: NM_133238.5) sequence, the positions and sequences of each exon and intron were determined. The BAC Clone containing the entire DC-SIGN gene: RP23-12K14 was purchased from the Children’hospotal Oakland Research Institute, and the BAC DNA was used HiPure Plasmid Filter Maxiprep Kit (Invitrogen) was prepared for use.
[0055] PCR amplification of the DC-SIGN 5' side homology arm (retrieval 5HA) and DC-SIGN 3' side homology arm (retrieval 3HA) of the target gene fragment: using RP23-12K14 BAC DNA as a template, using the forward primer DCSIGN-5HA -F(NotI)-ACCACC GCGGCCGC ACATCTGCCCATAGCACACAG (SEQ ID NO.1) and reverse primer DCSIGN-5HA-R (SpeI)-ACCACC ACTAGT AGCATCAG...
Embodiment 2
[0070] Example 2: Preparation of mouse embryonic stem (ES) cells targeted to insert IRES-DTR into DC-SIGN exon 7
[0071] Mouse embryonic fibroblasts (MEFs) culture: MEFs (ATCC) were cultured and maintained in MEF medium, passaged and frozen in time. MEF medium is DMEM medium with 10% FBS, 100U / ml penicillin streptomycin, 0.05mM 2mercaptoethanol, 2mML-glutamine.
[0072] Balb / c ES cell culture: MEFs cells were inactivated with 30 Gray γ-rays before use as trophoblast cells, Balb / c ES cells (Merck) were inoculated onto the inactivated MEFs, cultured and maintained with ES medium. ES cells were grown to 70% abundance and passaged at a ratio of 1:3. ES medium is DMEM medium with 15% FBS, 100U / ml penicillin, 1mM sodium pyruvate, 0.1mM non-essential amino acid, 0.05mM 2-mercaptoethanol, 2mM L-glutamine, 1μg / L leukemia inhibitory factor (LIF).
[0073] Electroporation of ES cells: ES cells were collected by trypsinization, washed once with PBS, resuspended in electroporation buff...
example 3
[0080] Example 3: Breeding DC SIGN-DTR transgenic mice with targeted insertion of IRES-DTR into DC-SIGN exon 7
[0081] Blastocyst injection of ES cells to obtain DC SIGN-DTR transgenic chimeric mice: Select 8-week-old well-developed C57BL / 6J male mice and C57BL / 6J female mice in a 1:2 cage, and select vaginal plug-positive females in the morning of the next day For mice, blastocysts can be obtained in the uterus if the female mouse is pregnant for 5 days. Ligated KM male mice and normal KM female mice were caged at a ratio of 1:2, and the female mice with vaginal plugs and swollen and ruddy vulva were selected as pseudopregnant KM female mice. The ES cells targeted to insert IRES-DTR to the DC-SIGN exon 7 site were injected into C57BL / 6J mouse blastocysts, and then inoculated into the uterus of pseudopregnant KM female mice, and chimeric mice were born ( F0), that is, cells derived from C57 and ES cells derived from BALB / c co-exist in the same individual, and the coat color ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com