Preparation method for co-producing bis(chlorosulfonyl)imide and lithium bis(fluorosulfonyl)imide
A technology for lithium bisfluorosulfonimide and bischlorosulfonimide acid is applied in the field of co-production of bischlorosulfonimide acid and lithium bisfluorosulfonimide, and can solve the problem of high product cost, fluorine Sulfonate isocyanates have problems such as high price and difficult industrial production, which can achieve the effects of easy operation, reduced consumption of raw and auxiliary materials, and fewer by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
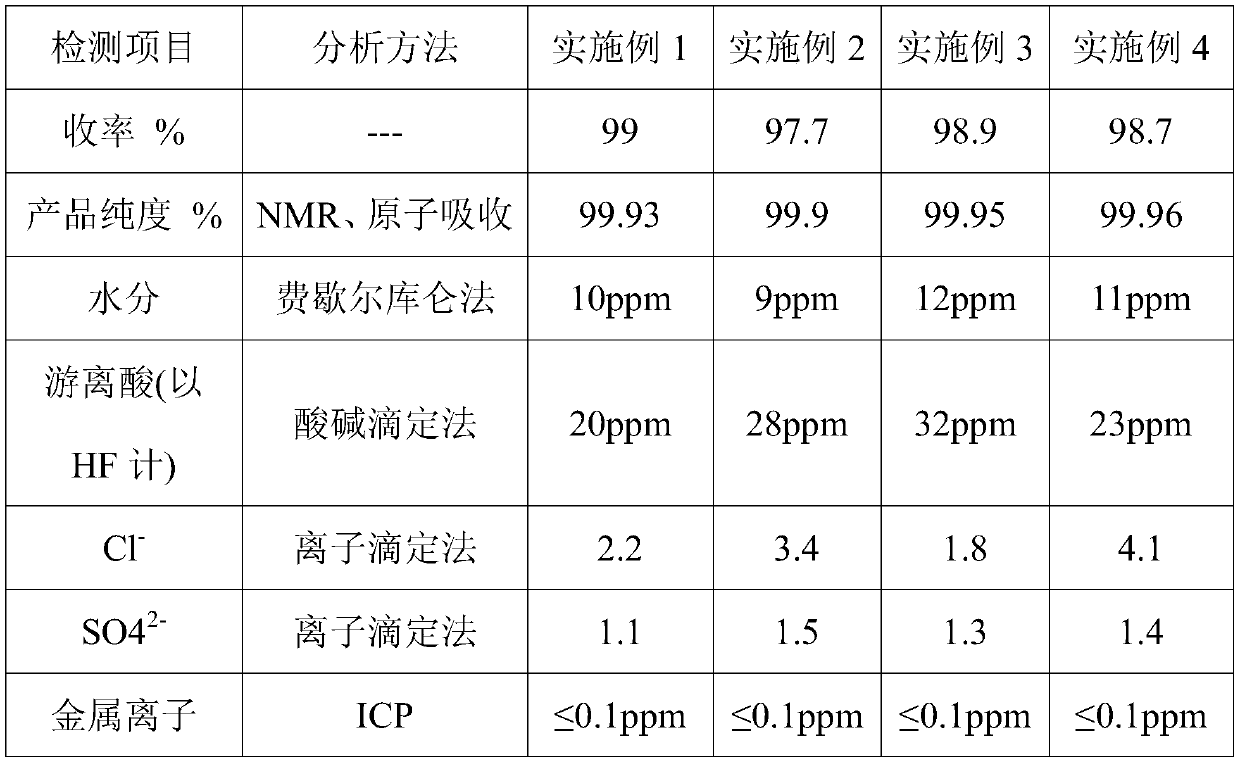
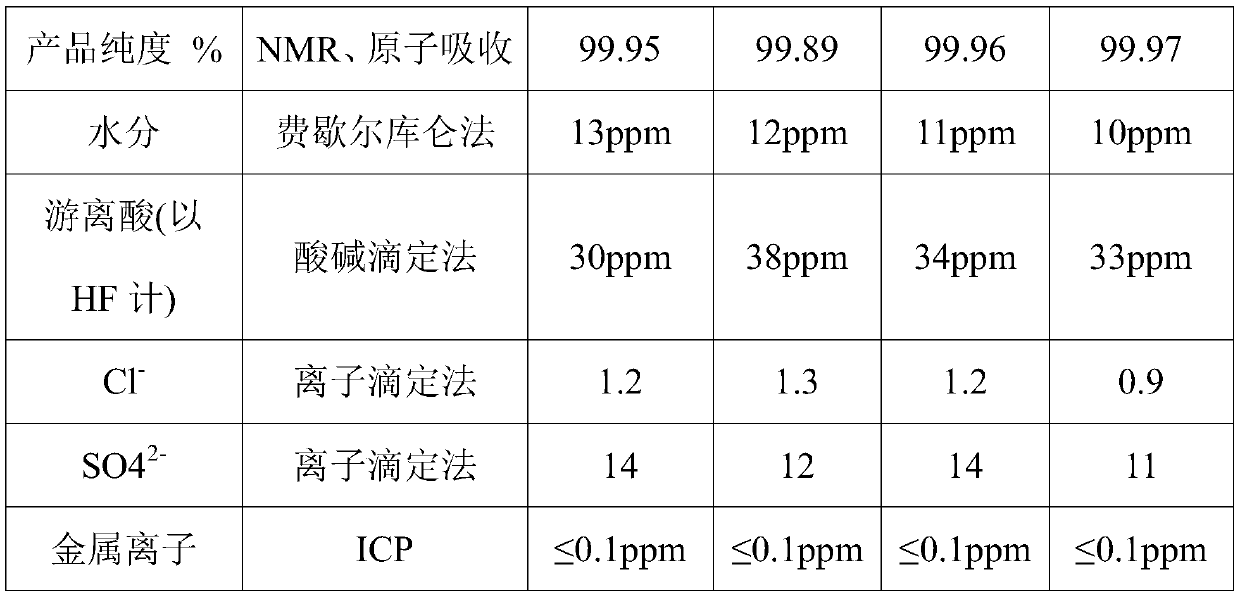
Embodiment 1
[0050] A preparation method for co-producing bischlorosulfonylimide acid and lithium bisfluorosulfonylimide, comprising the steps of:
[0051] S1. Add 0.25L of anhydrous acetonitrile into the PTFE-lined stainless steel reaction kettle, then add 135g of sulfuryl chloride, and slowly add 73.2g of octamethylcyclotetrasilazane with a pneumatic diaphragm pump while stirring. Stir the reaction at ℃ for 2 hours, and recover the remaining sulfuryl chloride and the by-product octamethylchlorosilane respectively by distillation under pressure. After recovery, acetonitrile is recovered by distillation under reduced pressure to obtain 53 g of dichlorosulfonylimide acid;
[0052] S2. Add 53.5g of bischlorosulfimidic acid and 0.042g of antimony pentachloride into the PTFE-lined stainless steel reaction kettle, raise the temperature to 90°C, slowly introduce 10g of anhydrous hydrofluoric acid gas under stirring, and cool down after 20 hours of reaction to room temperature, blowing nitrogen f...
Embodiment 2
[0055] A preparation method for co-producing bischlorosulfonylimide acid and lithium bisfluorosulfonylimide, comprising the steps of:
[0056] S1. Add 0.2L of N,N-dimethylformamide to the PFA-lined stainless steel reaction kettle, then add 135g of sulfuryl chloride, slowly add 146.4g of octamethylcyclotetrasilazane with a pneumatic diaphragm pump while stirring, drop After the addition was completed, the reaction was stirred at 30°C for 12 hours, and the remaining sulfuryl chloride and the by-product octamethylchlorosilane were respectively recovered by pressure distillation. After recovery, acetonitrile was recovered by vacuum distillation to obtain 104.6 g of dichlorosulfonylimide acid ;
[0057] S2. Add 107g of bischlorosulfimidic acid and 0.084g of antimony pentachloride to the PTFE-lined stainless steel reaction kettle, heat up to 110°C, slowly introduce 20g of anhydrous hydrofluoric acid gas under stirring, and cool down to At room temperature, blow nitrogen for 15 hour...
Embodiment 3
[0060] A preparation method for co-producing bischlorosulfonylimide acid and lithium bisfluorosulfonylimide, comprising the steps of:
[0061] S1. In the PTFE-lined stainless steel reaction kettle, add 0.1L ethyl acetate, then add 135g of sulfuryl chloride, and slowly add 117.1g of octamethylcyclotetrasilazane dropwise with a pneumatic diaphragm pump while stirring. Stir and react at ℃ for 8 hours, and recover the remaining sulfuryl chloride and the by-product octamethylchlorosilane respectively by distillation under pressure. After recovery, acetonitrile is recovered by distillation under reduced pressure to obtain 84.7 g of dichlorosulfonylimide acid;
[0062] S2. Add 85.6g of bischlorosulfimidic acid and 0.067g of antimony pentachloride into the PTFE-lined stainless steel reaction kettle, raise the temperature to 95°C, slowly introduce 16.8g of anhydrous hydrofluoric acid gas under stirring, and react for 18 hours Cool down to room temperature, blow nitrogen for 15 hours to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com