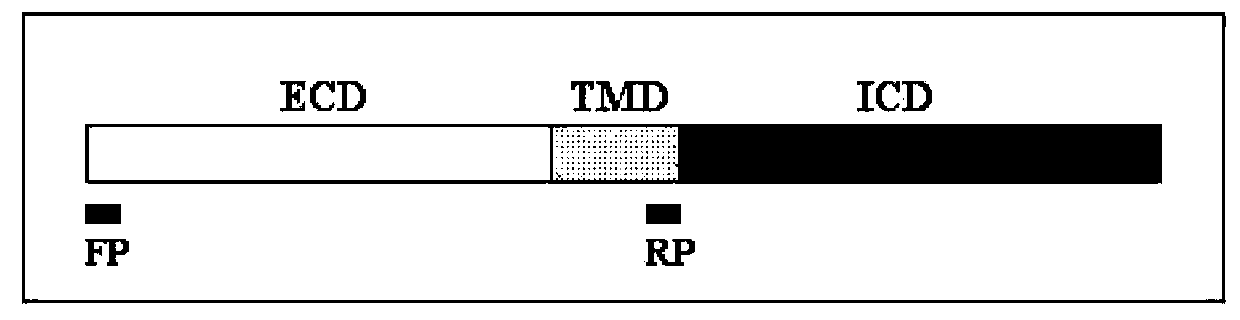
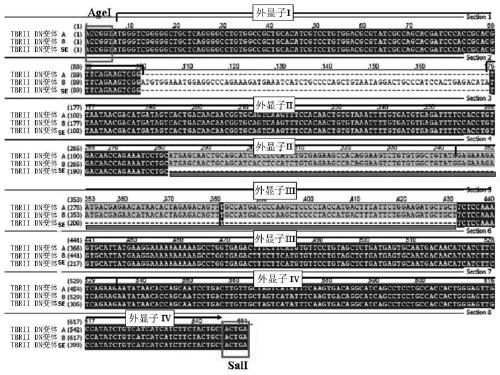
TGF-Beta receptor II isoform, fusion peptide, methods of treatment and methods in vitro
An isotype, RII-SE technology, used in fusion peptides, hybrid peptides, gene therapy, etc., to solve problems such as signal transduction disruption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1: Isolation, cloning and sequencing of TβRII-SE isoforms
[0116]Human adipose-derived mesenchymal stromal cells (hASCs) were obtained from 20 g of subcutaneous fat according to the protocol described by Zuk et al. and 1% L-glutamine in DMEM. Epstein Barr virus immortalized lymphoblasts were generated from peripheral blood mononuclear cells as described in "Protocols in Immunology" and cultured in RPMI medium. Human A459 (lung adenocarcinoma), HT1080 (fibrosarcoma), Caco-2 (colorectal cancer), Hep 3B (hepatocellular carcinoma), Jurkat (acute lymphoblastic leukemia), HEK293 (human embryonic kidney) and 293T cell lines. Place cells in humidified 5% CO 2 Cultured at 37°C in an incubator.
[0117] Purification of different leukocyte subsets
[0118] Granulocytes, lymphocytes and monocytes were isolated from heparinized peripheral blood by Ficoll-Paque™ PLUS (GE Healthcare Bio-Sciences AB) gradient centrifugation. After centrifugation, two fractions were obtai...
Embodiment 2
[0121] Example 2: Cloning of codon-optimized (co)TβRII-SE / Fc isotype fusion constructs
[0122] The TβRII-SE coding sequence containing the AgeI site was codon-optimized, the stop codon was deleted, and the Kozak sequence (Epoch Biolabs Inc. Texas, USA) was included. Use specific oligonucleotides as primers (forward: 5'AGA TCT GAC AAA ACT CAC ACA TGC 3' (SEQ ID No. 8) and reverse: 5'GAT ATC TTT ACCCGG AGA CAG G 3' (SEQ ID No.9)), the human IgG1 Fc coding sequence was obtained from total blood mRNA by RT-PCR, and the primers contained BglII site (forward primer) and EcoRV (reverse primer) to allow integration with TβRII-SE and Lentiviral vector in-frame fusion. The 951 bp AgeI / EcoRV fusion construct (coTβRII-SE / Fc) comprised a 258 bp coTβRII-SE fused in-frame to a 693 bp human IgG1-Fc.
Embodiment 3
[0123] Embodiment 3: lentiviral vector
[0124] The cDNAs encoding these three human TβRII isoforms were cloned into the pRRLsin18.cPPT.WPRE lentiviral vector, resulting in transfer vectors pRRLsin18.cPPT.CMV-TβRII-SE.ireseGFP.WPRE, pRRLsin18.cPPT.CMV-TβRII-DN .ireseGFP.WPRE and pRRLsin18.cPPT.CMV-coTβRII-SE / Fc.ireseGFP.WPRE. As previously described (R.A. Dewey et al., Experimental Hematology 34:1163-1171, 2006), by combining the transfer vector with an envelope plasmid (pCMV-VSVG), a packaging plasmid (pMDLg / pRRE) and a Rev plasmid (pRSV-REV) Transient transfection into the 293T cell line produced vesicular stomatitis virus G-pseudotyped lentivirus (VSV-G). Supernatants were collected every 12 hours for 48 hours and frozen in aliquots. Virus titers were determined by transduction of A549 cells (produced 10 7 infection particles). The pRRLsin18.cPPT.CMV-eGFP.WPRE lentiviral vector was used as a control.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com