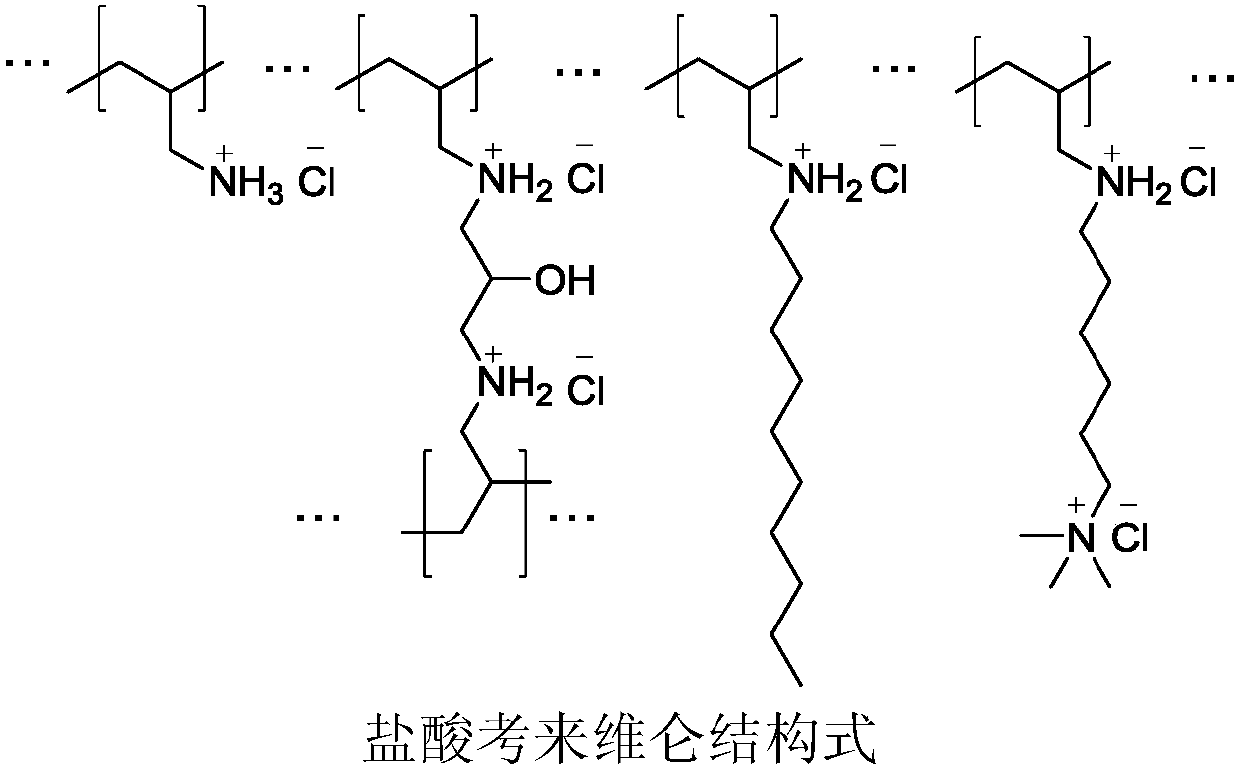
Medicine composition with colesevelam hydrochloride and preparation medicine of medicine composition
A technology of colesevelam hydrochloride and colesevelam, applied in the direction of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as poor stability and low content uniformity, and overcome poor fluidity, Good reproducibility and good drug content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
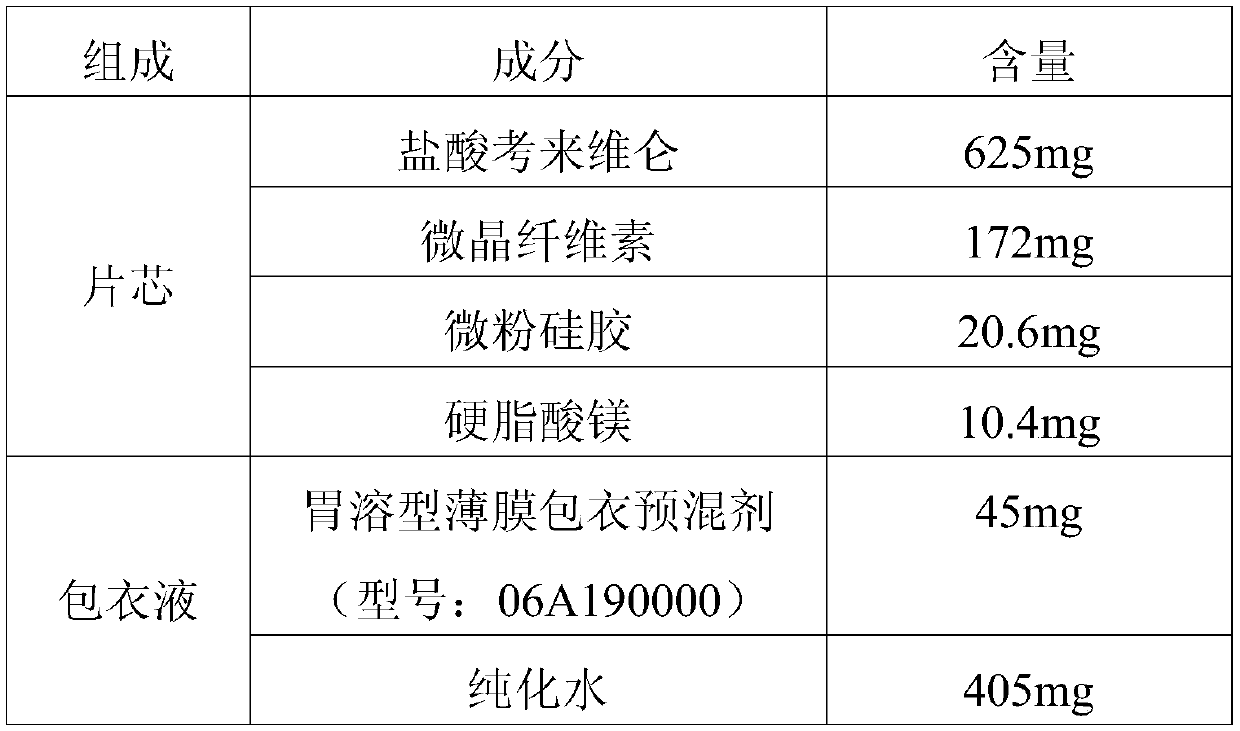
[0038] Prepare the pharmaceutical composition according to the prescription in Table 1.
[0039] Table 1
[0040]
[0041] Preparation Process:
[0042] (1) Part of colesevelam hydrochloride (accounting for 15% of the total mass) and micropowdered silica gel were mixed and stirred at a rotation speed of 280r for 10 minutes, and then passed through a 40-mesh sieve to obtain a sieved mixture;
[0043] (2) The remaining colesevelam hydrochloride and the sieved mixture were mixed and stirred for 30 minutes at a rotation speed of 280r to obtain premix 1;
[0044] (3) Perform dry granulation on premix 1, and mix the obtained granules with microcrystalline cellulose for 30 minutes to obtain premix 2;
[0045] (4) Add the magnesium stearate that crosses 40 mesh sieves in the premix 2, after mixing 10min, obtain total mixture;
[0046] (5) Tablets were pressed with a rotary tablet press with different process parameters, and finally prepared by coating and printing at 30°C.
[0...
Embodiment 2
[0051] Prepare pharmaceutical composition according to table 3 prescription.
[0052] table 3
[0053]
[0054]
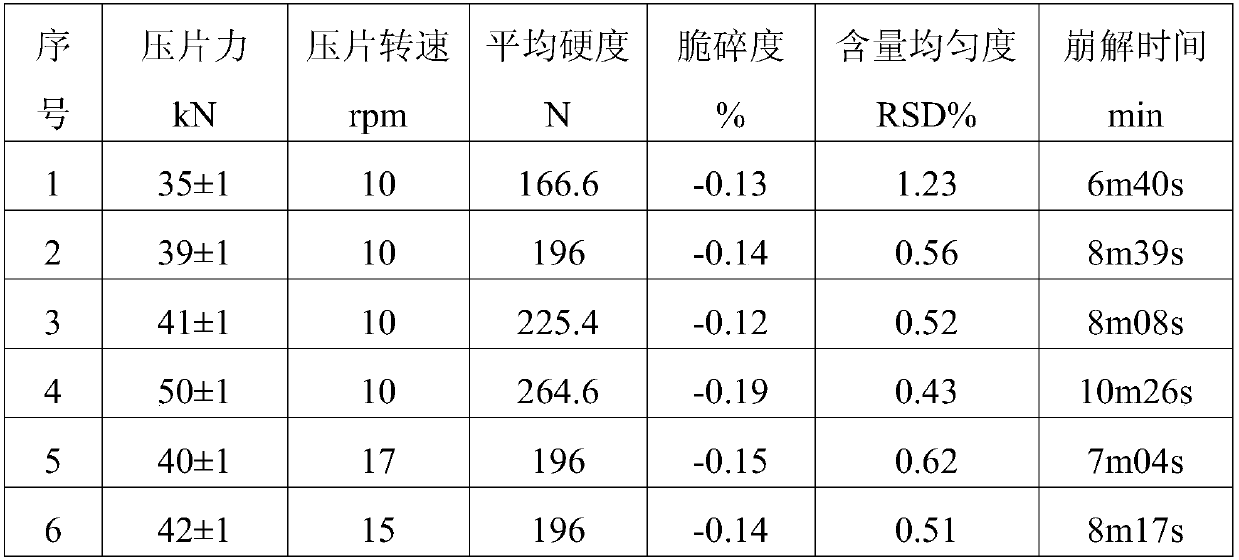
[0055] The preparation method is the same as in Example 1, wherein part of colesevelam hydrochloride accounts for 25% of the total mass, the stirring speed in steps (1) and (2) is 330r, and the tabletting pressure is 41 ± 1KN, and the tabletting speed is 41 ± 1KN. Tablet compression is carried out under the condition of 10 rpm, and the coating temperature is 38°C. After testing, the average hardness is 213N, the friability is -0.18%, the content uniformity is 0.70%, and the disintegration time is 9m13s.
Embodiment 3
[0057] Prepare pharmaceutical composition according to table 4 prescription.
[0058] Table 4
[0059]
[0060] The preparation method is the same as in Example 1, wherein part of colesevelam hydrochloride accounts for 35% of the total mass, the stirring speed in steps (1) and (2) is 300r, and the tabletting pressure is 41 ± 1KN, and the tabletting speed is 41 ± 1KN. Tablet compression is carried out under the condition of 10 rpm, and the coating temperature is 35°C. After testing, the average hardness is 169N, the friability is -0.14%, the content uniformity is 0.59%, and the disintegration time is 6m10s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com