Method for reducing nitrocompound through biocatalysis
A technology for nitro compounds and biocatalysis, which is applied in the field of biocatalytic reduction of nitro compounds, can solve the problems of difficult separation and treatment of products, potential safety hazards, and heavy use of heavy metals, etc., achieves high-throughput and rapid nitro reduction reaction, and is easy to operate. Fast, broad substrate availability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
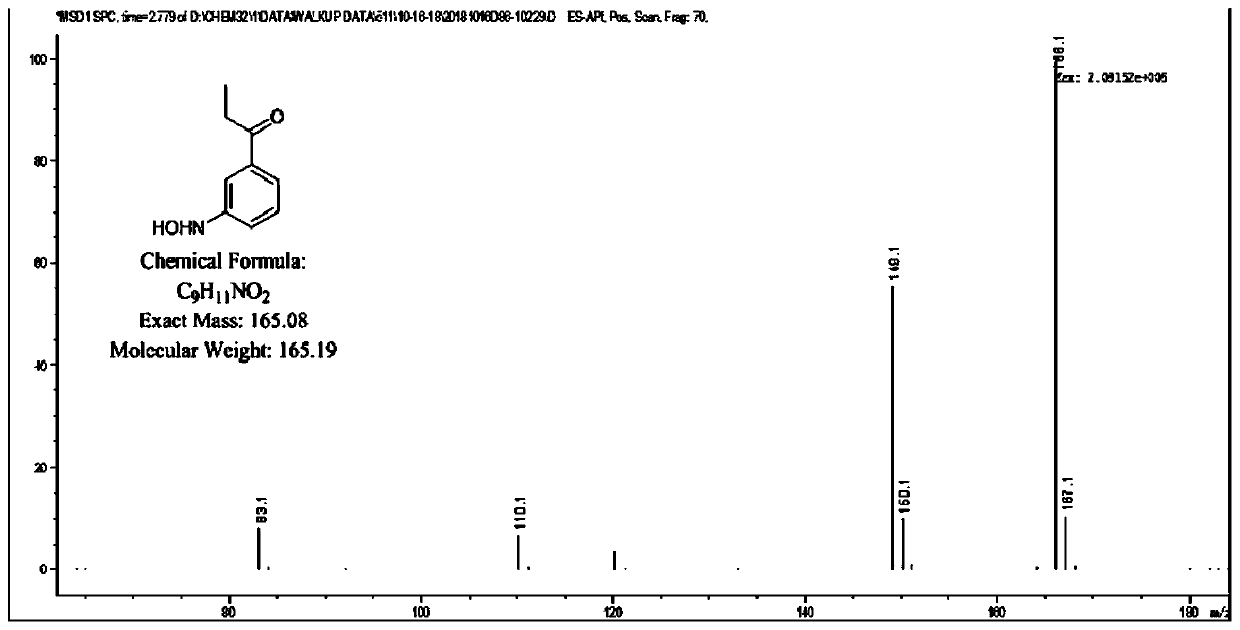
[0044] Take 0.25g m-nitropropiophenone (3-nitropropiophenone), 10mL dimethyl sulfoxide (10% v / v), 2g glucose (20g / L), 1g olefin reductase ERED-103, 0.1g glucose Dehydrogenase, 1mL Triton X-100, 5mg NADP + After mixing with 89mL sodium phosphate buffer solution (100mM, pH7.0), react at 30°C with a shaker at 300r / min for 24h. After the reaction, take a sample and add ethanol, centrifuge, and collect the filtrate by filtration to prepare Hydroxylamine compound. LC-MS is used for analysis, and it is measured that the conversion rate of the nitro compound into the hydroxylamine compound is 95%.
[0045]
[0046] In this embodiment, glucose dehydrogenase, NADP + , Glucose is mainly used for the preparation and regeneration of NADPH (reduced coenzyme II), to construct an efficient coenzyme NADPH regeneration system; NAD can also be used + , used to prepare coenzyme NADH and construct its regeneration system.
[0047] In this embodiment, the cosolvent can also be isopropanol, 1...
Embodiment 2
[0050] Take 0.25g p-nitrotoluene (4-nitrotoluene), 10mL dimethyl sulfoxide (10% v / v), 2g glucose (20g / L), 1g olefin reductase ERED-103, 0.1g glucose dehydrogenation Enzyme, 1mL Triton X-100, 5mg NADP + After mixing with 89mL sodium phosphate buffer solution (100mM, pH7.0), react at 30°C with a shaker at 300r / min for 24h. After the reaction, take a sample and add ethanol, centrifuge, and collect the filtrate by filtration to prepare Hydroxylamine compound. The hydroxylamine compound is 4-(hydroxyamino)toluene.
[0051]
[0052] In this embodiment, glucose dehydrogenase, NADP + , Glucose is mainly used for the preparation and regeneration of NADPH (reduced coenzyme II), to construct an efficient coenzyme NADPH regeneration system; NAD can also be used + , used to prepare coenzyme NADH and construct its regeneration system.
[0053] In this embodiment, the cosolvent can also be isopropanol, 1,3-dioxane or ethyl acetate; the buffer can also be trimethylolmethylamine-hydroch...
Embodiment 3
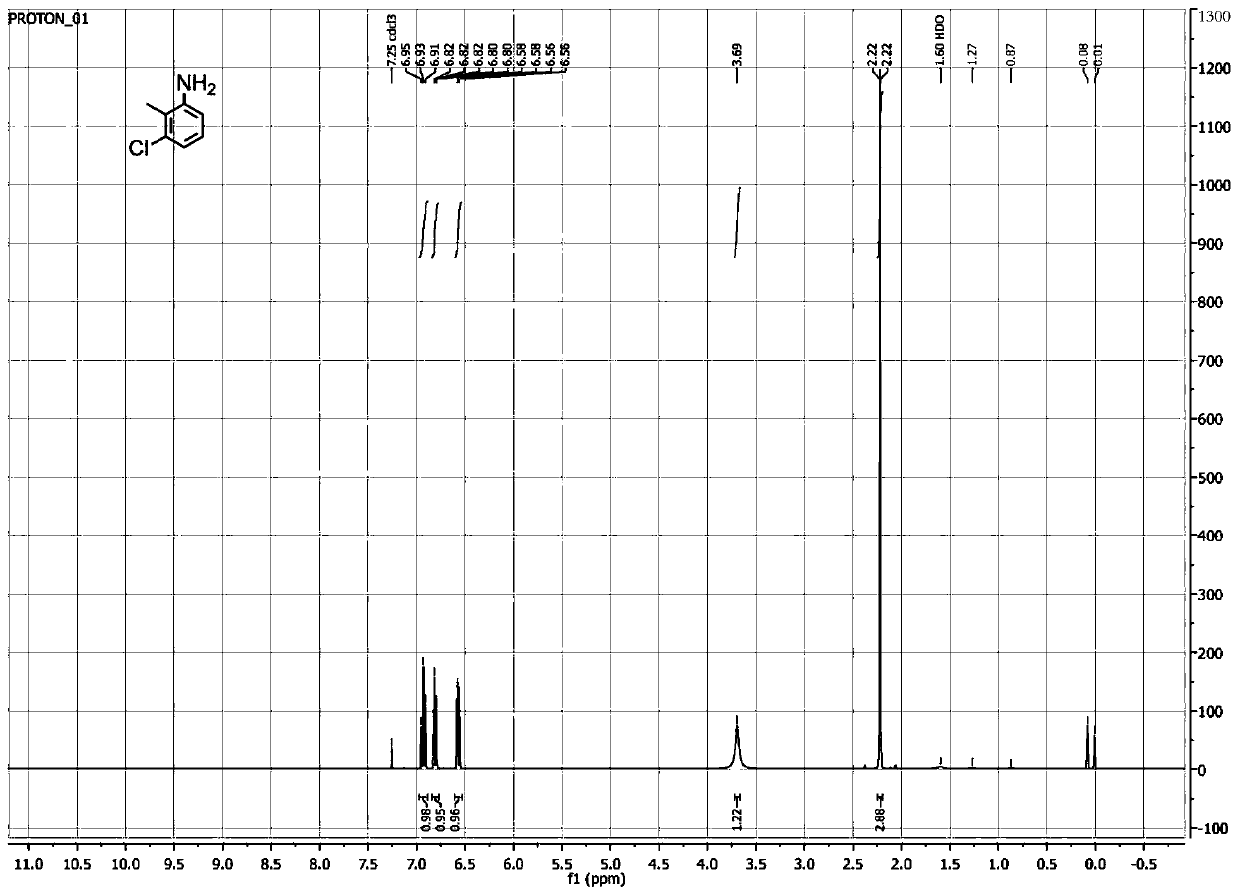
[0056] Take 0.25g 1-chloro-2-methyl-3-nitrobenzene, 10mL dimethyl sulfoxide (10% v / v), 2g glucose (20g / L), 1g olefin reductase ERED-103, 0.1g glucose Dehydrogenase, 1mL Triton X-100, 5mg NADP + After mixing with 89mL sodium phosphate buffer solution (100mM, pH7.0), react at 30°C with a shaker at 300r / min for 24h. After the reaction, take a sample and add ethanol, centrifuge, and collect the filtrate by filtration to prepare Hydroxylamine compound. The hydroxylamine compound is 3-chloro-2-methylphenylhydroxylamine.
[0057]
[0058] In this embodiment, glucose dehydrogenase, NADP + , Glucose is mainly used for the preparation and regeneration of NADPH (reduced coenzyme II), to construct an efficient coenzyme NADPH regeneration system; NAD can also be used + , used to prepare coenzyme NADH and construct its regeneration system.
[0059] In this embodiment, the cosolvent can also be isopropanol, 1,3-dioxane or ethyl acetate; the buffer can also be trimethylolmethylamine-hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com