Methylprednisolone sodium succinate for injection and preparation method thereof
A technology of methylprednisolone sodium succinate and methylprednisolone succinate, applied in the field of medicine, can solve the problems of shortened freeze-drying time, low production efficiency, and affecting the quality of preparations, so as to shorten the freeze-drying time, improve production efficiency, and improve appearance uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
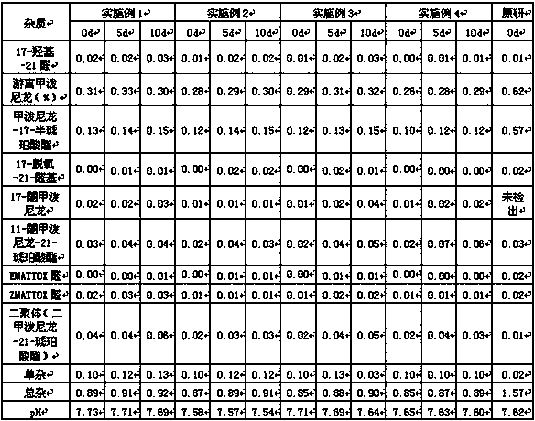
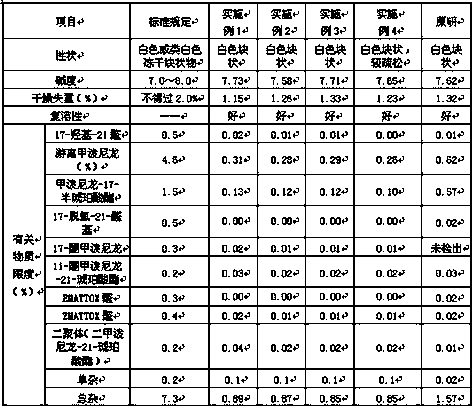
Examples
Embodiment 1
[0039] Methylprednisolone sodium succinate for injection, its specification is 125mg according to methylprednisolone, and the prescription composition of each is as follows:
[0040] Methylprednisolone succinate 158.4mg
[0041] Sodium dihydrogen phosphate 1.8mg
[0042] Disodium hydrogen phosphate 17.5mg
[0043] Sodium hydroxide 13.4mg
[0044] Mannitol 0mg
[0045] Lactose 0mg
[0046] Water for injection, fixed weight to 1.5g
[0047] The preparation process of medicinal liquid comprises the following steps:
[0048] (1) Prepare the prescribed amount of sodium hydroxide into lye with a concentration of 10% for later use,
[0049] (2) Add water for injection with 70% of the total volume of the liquid into the liquid preparation tank, cool down to 15°C,
[0050] (3) Add the prescribed amount of sodium dihydrogen phosphate and disodium hydrogen phosphate into the liquid mixing tank, stir to dissolve,
[0051] (4) Add the prescribed amount of methylprednisolone succina...
Embodiment 2
[0065]Methylprednisolone sodium succinate for injection, its specification is recorded as 40mg by methylprednisolone, and the prescription composition of each is as follows:
[0066] Methylprednisolone Succinate 50.7mg
[0067] Sodium dihydrogen phosphate 1.84mg
[0068] Disodium hydrogen phosphate 17.5mg
[0069] Sodium hydroxide 4.27mg
[0070] Mannitol 0mg
[0071] Lactose 25mg
[0072] Water for injection, fixed weight to 1g
[0073] The preparation process of medicinal liquid comprises the following steps:
[0074] (1) Prepare the prescribed amount of sodium hydroxide into lye with a concentration of 10% for later use,
[0075] (2) Add water for injection with 60% of the total amount of liquid in the liquid preparation tank, cool down to 15°C,
[0076] (3) Add the prescribed amount of sodium dihydrogen phosphate and disodium hydrogen phosphate into the liquid mixing tank, stir to dissolve,
[0077] (4) Add the prescribed amount of methylprednisolone succinate into...
Embodiment 3
[0092] Methylprednisolone sodium succinate for injection, its specification is recorded as 40mg by methylprednisolone, and the prescription composition of each is as follows:
[0093] Methylprednisolone Succinate 50.7mg
[0094] Sodium dihydrogen phosphate 1.84mg
[0095] Disodium hydrogen phosphate 17.5mg
[0096] Sodium hydroxide 4.27mg
[0097] Mannitol 25mg
[0098] Lactose 0mg
[0099] Water for injection fixed to 1g
[0100] The preparation process of medicinal liquid comprises the following steps:
[0101] (1) Prepare the prescribed amount of sodium hydroxide into lye with a concentration of 10% for later use,
[0102] (2) Add water for injection with 60% of the total amount of liquid in the liquid preparation tank, cool down to 15°C,
[0103] (3) Add the prescribed amount of sodium dihydrogen phosphate and disodium hydrogen phosphate into the liquid mixing tank, stir to dissolve,
[0104] (4) Add the prescribed amount of methylprednisolone succinate into the li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com