Application method of aFe(OH)xOy/Pt catalyst as hydrogenation reaction catalyst
An application method, hydrogenation reaction technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve the problems of low catalytic efficiency and high hydrogen pressure, and achieve High conversion rate of nitro group, low reaction temperature, good amination selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
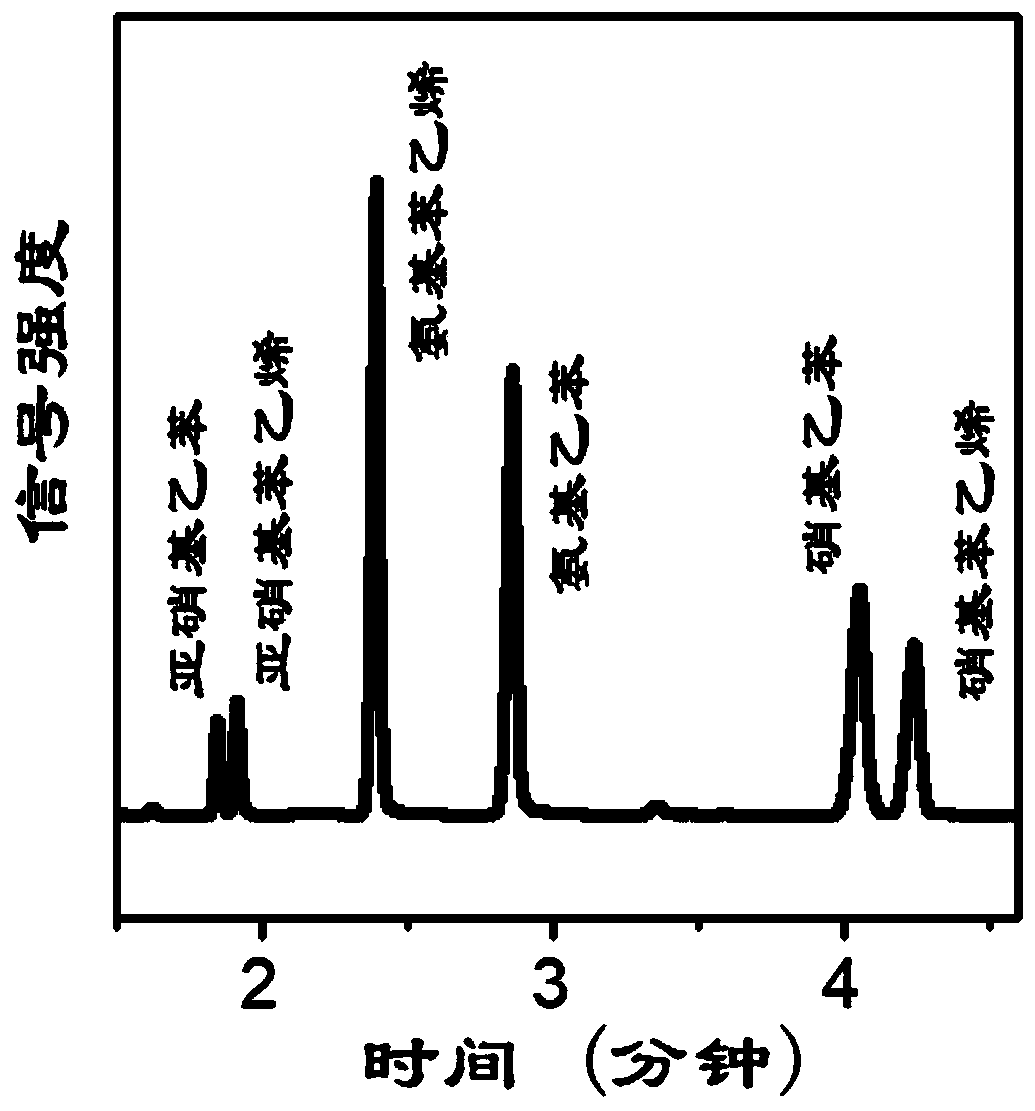
[0031] aFe(OH) x o y / Pt catalyst is used as the application method of hydrogenation reaction catalyst, in container, add organic solvent, the nitrobenzene compounds shown in general formula (1) and aFe(OH) x o y / Pt catalyst, mix evenly, raise the temperature to 50-75°C, pass in hydrogen to react, the reaction time is 30-200min, stop the flow of hydrogen, and lower the temperature;
[0032]
[0033] Among them, the catalyst is incompletely modified Fe(OH) on the surface of Pt x o y , that is, part of the exposed surface of the catalyst is Pt, and the other part is Fe(OH) x o y ;
[0034] a is the molar ratio of Fe and Pt, 0.01≤a≤0.5, x+2y=3, x / y=0.34-0.89, R is H or a substituent, and R is not an amino group.
[0035] Preferably, the aFe(OH) x o y The / Pt catalyst is loaded on the carrier to form a supported catalyst.
[0036] More preferably, the mass percentage of Pt in the supported catalyst is 0.5-5%. More preferably, the mass percentage of Pt in the support...
Embodiment 1
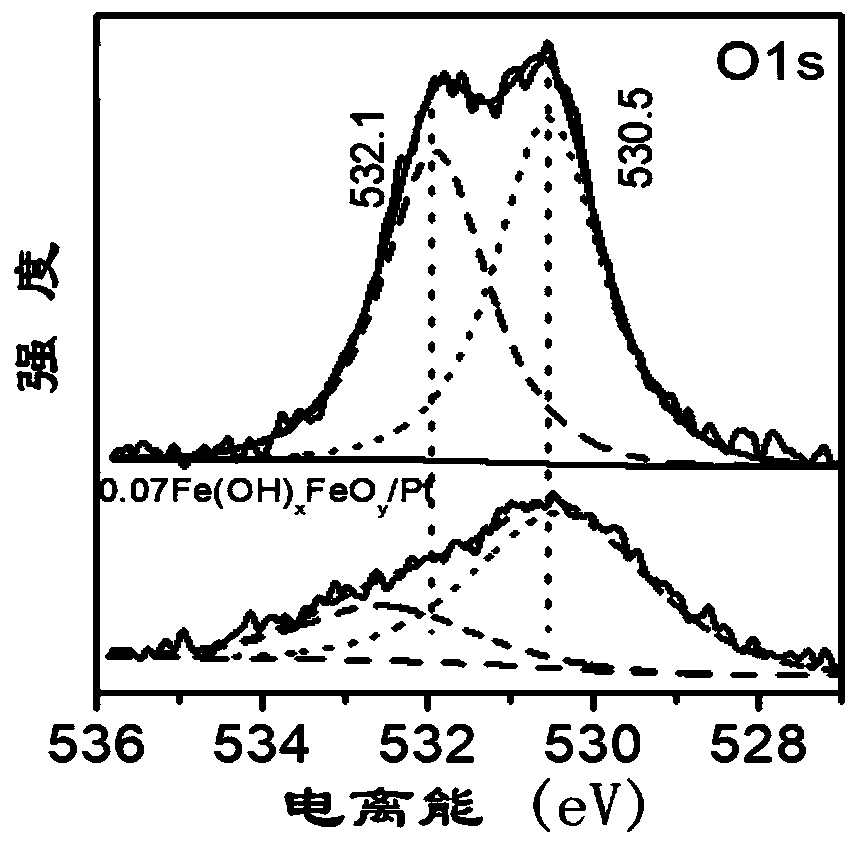
[0045] Add 1.5 mg of iron acetylacetonate to 10 mg of Pt nanoparticles protected by oleylamine, react at 240° C. for 1 h in 1 atmosphere of CO, cool down to room temperature, and wash to obtain Catalyst 1. figure 1 It is the XPS spectrum of catalyst 1. attached figure 1 The peak positions and peak areas are shown in Table 1.
[0046] Table 1
[0047]
[0048] Therefore, the expression for Catalyst 1 is 0.07Fe(OH) 0.44 o 1.28 / Pt.
Embodiment 2
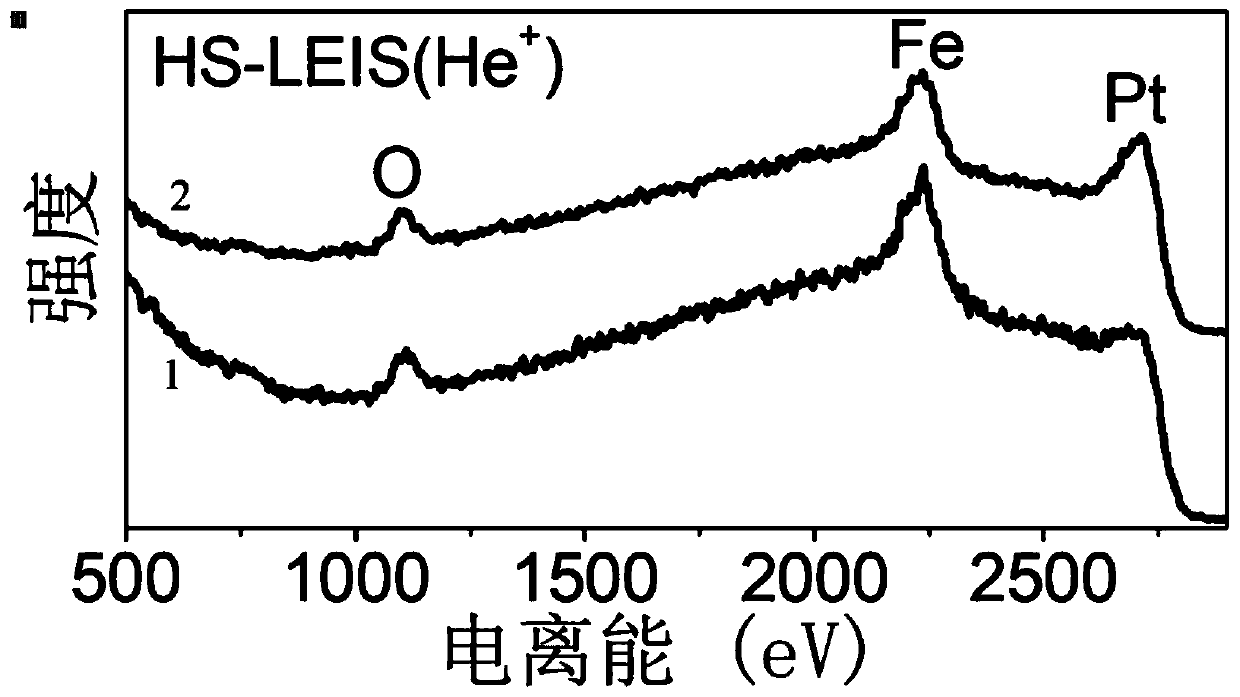
[0050] Add 2.8 mg iron acetylacetonate to 10 mg Pt nanoparticles protected by oleylamine, react at 240 degrees, 1 atmospheric pressure of CO for 1 h, drop to room temperature, wash, and load on activated carbon according to the mass fraction of Pt as 2%, to obtain Supported Catalyst 2. The expression for catalyst 2 is 0.19Fe(OH) 0.7 o 1.15 / Pt.
[0051] attached figure 2 It is the low-energy ion scattering spectrum of catalyst 2. There are three elements of O, Fe and Pt on the surface of catalyst 2. After the particles of catalyst 2 are sputtered by 5keV ion beam for 5min, the peak intensity of Pt is obviously enhanced, which proves that the Fe on the surface of catalyst 2 particles is The ion beam is bombarded to expose the internal Pt. The above shows that the structure of catalyst 2 is that the surface of Pt is not completely modified with Fe(OH) x o y .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com