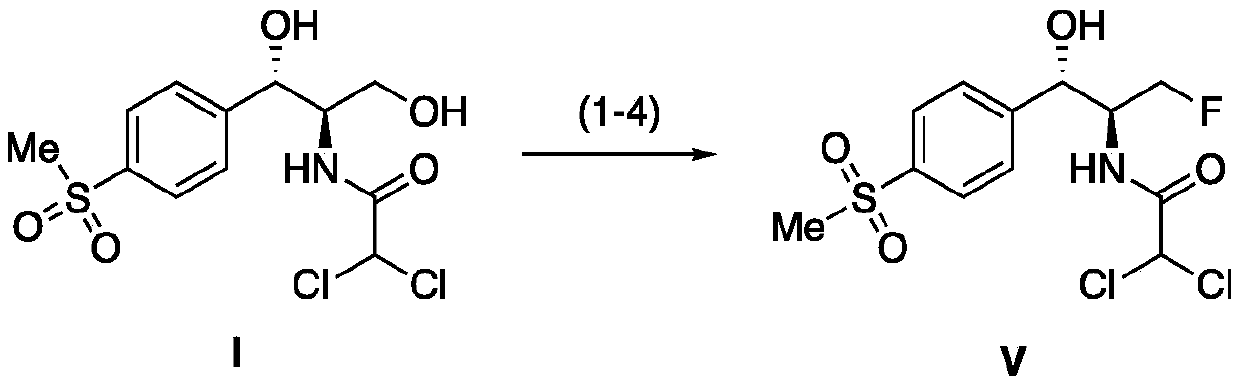
Synthesis method of florfenicol
A technology of florfenicol and synthetic method, which is applied in the field of veterinary drug preparation, can solve the problems of high cost of hydroxyl activation reagents, and achieve the effects of mature processing technology, low processing difficulty and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
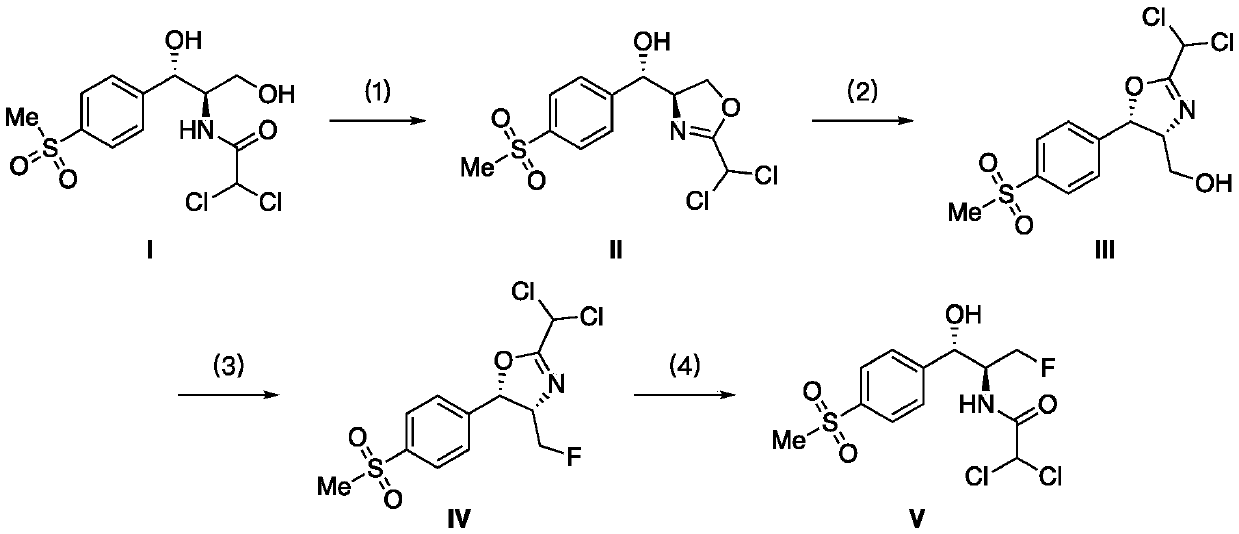
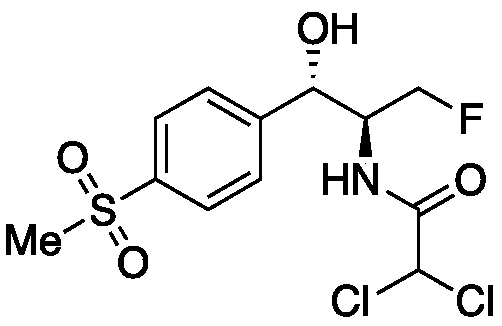
[0037] in N 2 Under gas protection, dissolve compound I (2.05g, 5.8mmol) in 35mL of dichloromethane, add triethylamine (acid-binding agent 1, 1.17g, 2.0equiv), replace sulfuryl fluoride gas, and adjust sulfuryl fluoride The pressure was 0.2 MPa, stirred at room temperature, and detected by TLC until the reaction was completed. Add 10 mL of saturated K to the reaction solution 2 CO 3 The solution was quenched, added dichloromethane (2×60mL) for extraction, combined the organic phases, and washed with saturated K 2 CO 3 Washing (2×60 mL), washing with saturated brine (40 mL), drying over anhydrous sodium sulfate, and column chromatography gave Compound II (1.6320 g, 84%). Compound Characterization: 1 H NMR (500MHz, DMSO-d 6 )δ7.87(d, J=7.1Hz, 1H), 7.61(d, J=7.3Hz, 1H), 7.02(s, 1H), 5.88(d, J=3.5Hz, 1H), 4.86(s, 1H), 4.58(s, 1H), 4.47(t, J=8.8Hz, 1H), 4.40(t, J=7.4Hz, 1H), 3.19(s, 3H); 13 C NMR (126MHz, DMSO-d 6 )δ 162.02, 148.11, 139.35, 127.53, 126.40, 71.5...
Embodiment 2
[0039] The method is the same as in Example 1, except that the sulfuryl fluoride pressure is 0.1 MPa, and the reaction yield is 72%.
Embodiment 3
[0041] The method is the same as in Example 1, except that the acid-binding agent 1 is replaced by NaOH (348 mg, 1.5 equiv), the organic solvent is replaced by 1,4-dioxane, the reaction temperature is raised to 50 ° C, and the reaction yield is 73 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com