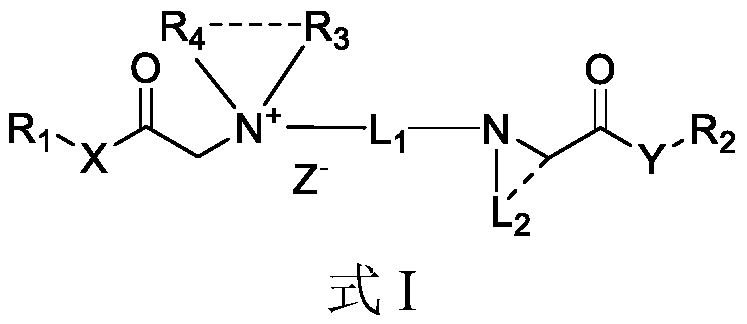
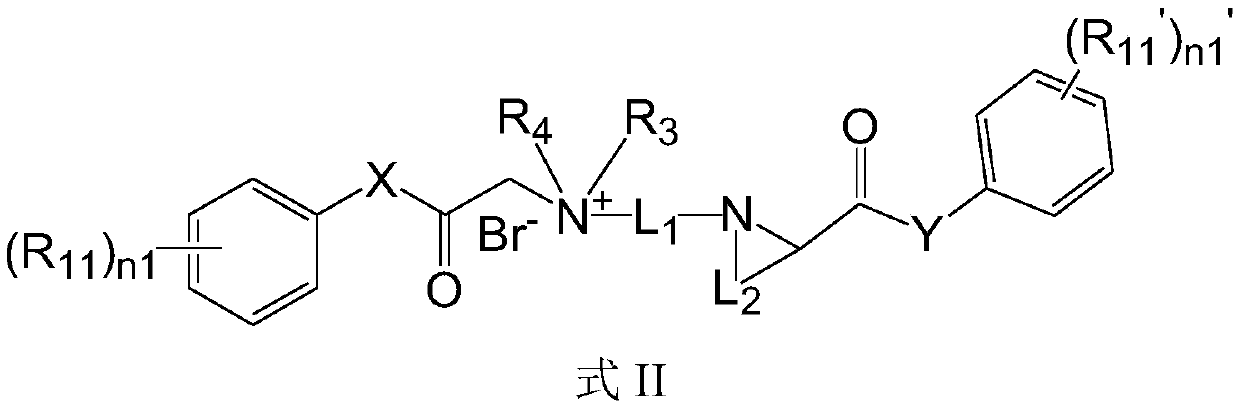
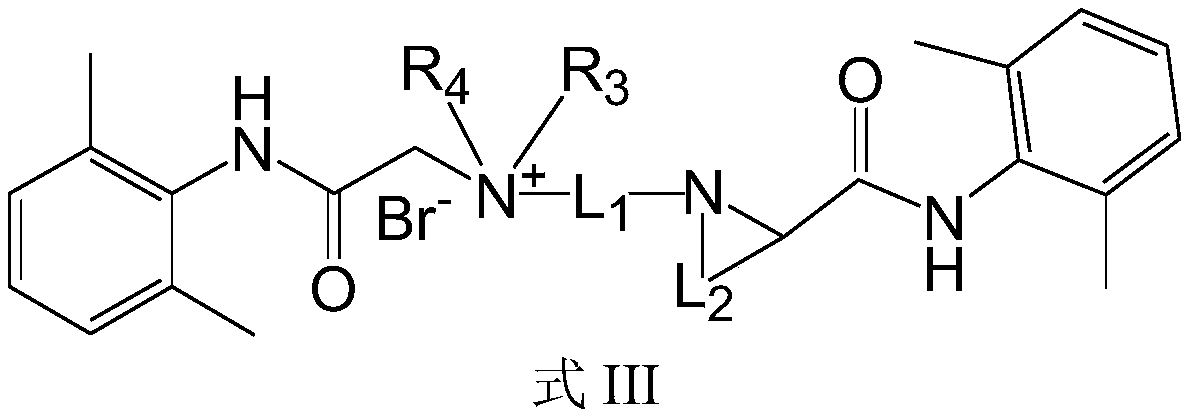
Quaternary ammonium salt compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of chemical medicine, can solve the problems of muscle and nerve injury, poor local anesthesia selectivity, and no selective local anesthetic effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1, the preparation of compound of the present invention
[0100]
[0101] Compound 1a (5.0g, 45.39mmol) was dissolved in 15mL of 1,3-dibromopropane, heated to 75°C for 40h, monitored by TLC (DCM:MeOH=10:1, R f = 0.3). Add an appropriate amount of ethyl acetate to form a viscous syrupy substance, pour off the supernatant, and the remaining 6g of crude product is dissolved in 30mL of methanol and then mixed with silica gel. After dry loading, the sample is purified by silica gel column chromatography. The eluent is CH 2 Cl 2 : MeOH=10:1, the eluate was collected and concentrated to obtain 3 g of crude product. Ethyl acetate and dichloromethane were recrystallized to obtain 2.5 g of off-white solid powder (1b), yield: 31.6%, which was used for the next reaction.
[0102] Intermediate 1b (2.00g, 3.66mmol) and N-(2,6-dimethylphenyl)-2-piperidinecarboxamide (0.934g, 4.03mmol, CAS: 15883-20-2 ) was dissolved in 20mL of ethanol, DIPEA (0.94g, 1.21mL, 7.32mmol...
Embodiment 2
[0103] Embodiment 2, the preparation of compound of the present invention
[0104]
[0105] Compound 2a (2.0g, 40.32mmol) was dissolved in 5mL of bis(2-bromoethyl)ether, heated to 75°C for 24h, monitored by TLC (DCM:MeOH=10:1, R f = 0.3). An appropriate amount of ethyl acetate was added, and the reaction solution solidified to produce a white solid. 3 g of the crude product was filtered out and purified by silica gel column chromatography. The eluent is CH 2 Cl 2 :MeOH=20:1, the eluate was collected and concentrated to obtain 5.9g of white solid (2b), yield: 31.5%, which was used for the next reaction.
[0106]Intermediate 2b (1.0g, 2.16mmol) and N-(2,6-dimethylphenyl)-2-piperidinecarboxamide (0.55g, 2.37mmol, CAS: 15883-20-2) prepared above Dissolve in 15mL of ethanol, add DIPEA (0.53g, 0.68ml, 4.12mol), react at 30°C for 10 days, evaporate the solvent, and purify the crude product by silica gel column chromatography, the eluent is CH 2 Cl 2 : MeOH=10:1, the eluate w...
Embodiment 3
[0107] Embodiment 3, the preparation of compound of the present invention
[0108]
[0109] Compound 3a (500mg, 1.2mmol), 1,14-dibromotetradecane (2g, 6.0mmol) was dissolved in 5mL of acetonitrile, heated to 70°C for 24h, monitored by TLC (DCM:MeOH=20:1, R f = 0.3). An appropriate amount of ethyl acetate was added, and the reaction solution solidified to produce a white solid. The crude product, 0.9 g of the white solid, was filtered out and purified by silica gel column chromatography. The eluent is CH 2 Cl 2 : MeOH=20:1, the eluate was collected and concentrated to obtain 500 mg of white powdery solid (3b), yield: 54.1%, which was used for the next reaction.
[0110] Intermediates 3b (500mg, 0.65mmol) and 3c (0.18g, 0.71mmol) prepared above were dissolved in 30ml of ethanol and 5ml of methanol mixed solvent, DIPEA (0.17g, 0.21mL, 1.3mmol) was added, and reacted at 30°C for 10 sky. After the reaction was complete, it was purified by silica gel column chromatography. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com