Application of calycosin derivative to preparation of medicines for restraining proliferation of endothelial cells
An endothelial cell proliferation and drug technology, applied in the field of medicine, can solve the problems of large effective concentration and unclear target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of the compound represented by formula (I) used in the following experimental examples of this application:
[0028] Specifically synthesized according to the following synthetic route:
[0029]
[0030] Among them, the chemical name of compound 1 is: 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one, and compound 2 is ethyl chloroacetate.
[0031] The specific synthesis method is:
[0032] Take 1g (3.52mmol) of compound 1 (mullin isoflavone) in a 50ml round bottom flask and dissolve it with 40ml of acetone, then add 3g of anhydrous K 2 CO 3 And 0.5g of NaI, stirred at room temperature for 1h, then added 0.91g (7.43mmol) of compound 2 (ethyl chloroacetate) dropwise, then placed in a water bath at 45°C and stirred at reflux for 4h (TCL monitors the reaction, the developing solvent is chloroform: methanol=60: 1. Volume ratio); then stop heating and continue stirring until cooling. Add ice water to the cooled reaction, filter, collect the precipitate, dis...
experiment example 1
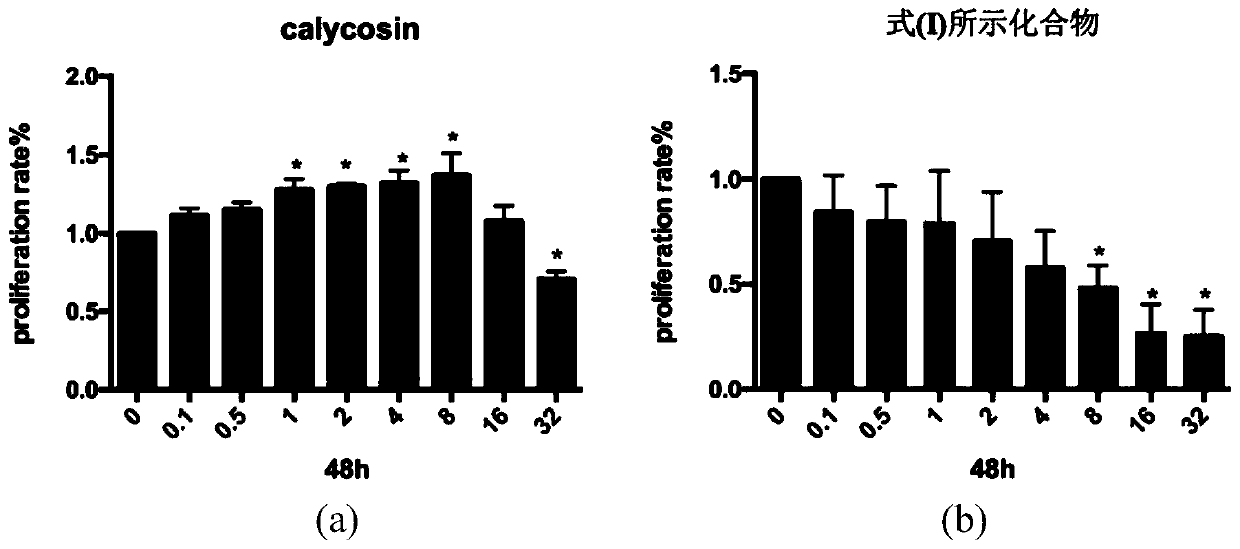
[0043] Experimental example 1: MTT method to detect endothelial cell proliferation
[0044] Take HUVECs that grow to 90% confluence, wash and digest them to prepare cell suspension. After counting, press 5x10 3 / Well inoculated in 96-well plate. After the cells were fully adhered to the wall after 24 hours of culture, the upper layer of culture medium was removed, and 100 μl of low serum hormone-free culture medium (0.5% CS-FBS) was added to each well and cultured for 24 hours to synchronize the cell status. The experimental group was set up with different concentrations of CAG002 (0.1μM~32μM) dosing groups, each group had 6 multiple wells, and 200μl / well was added with drug-containing low serum culture medium. At the same time, a control was set. The blank control group was 0.1% DMSO culture solution, and the positive control was Calycosin.
[0045] MTT results showed that as the concentration of CAG002 increased, its inhibitory effect on HUVECs gradually increased. When the dru...
experiment example 2
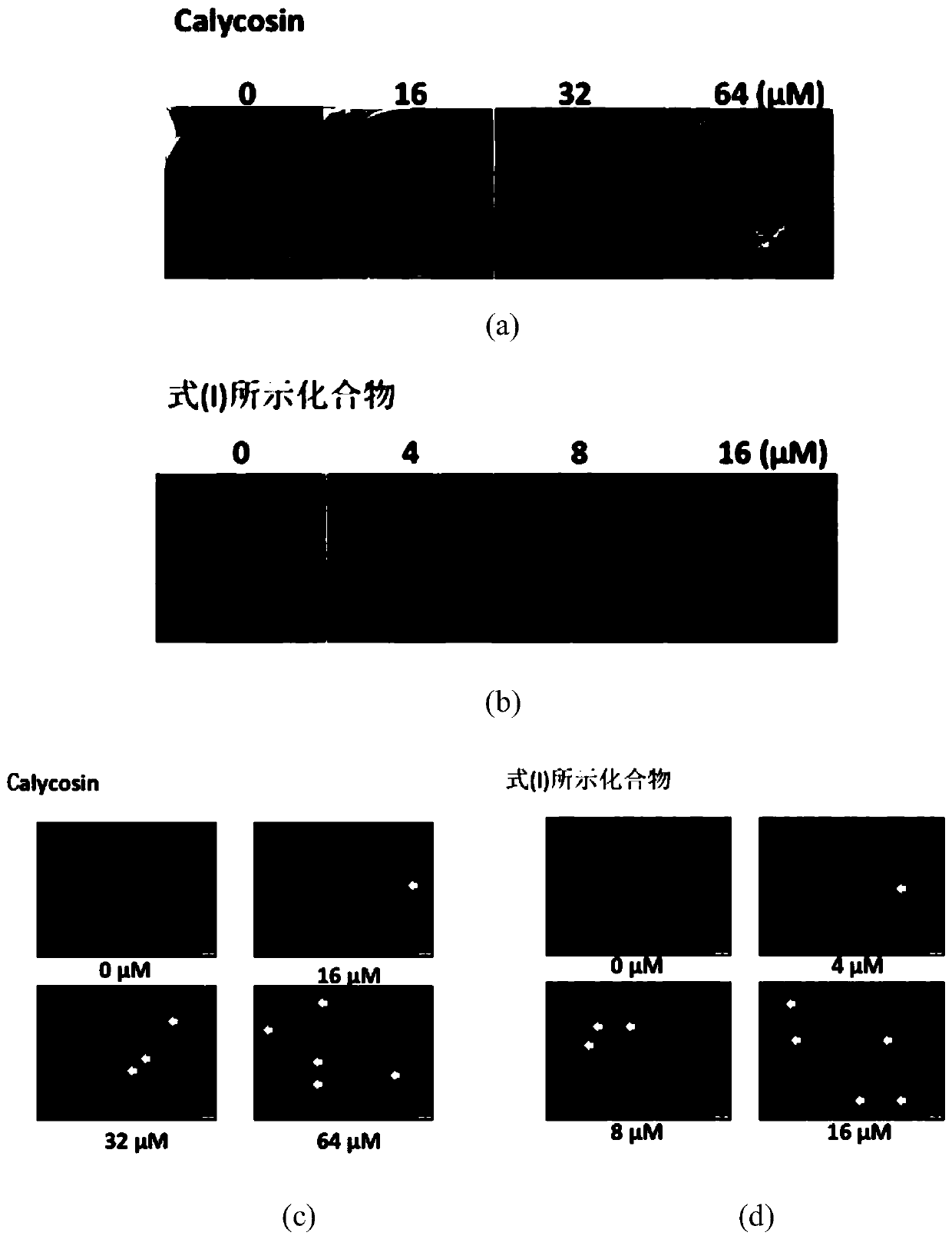
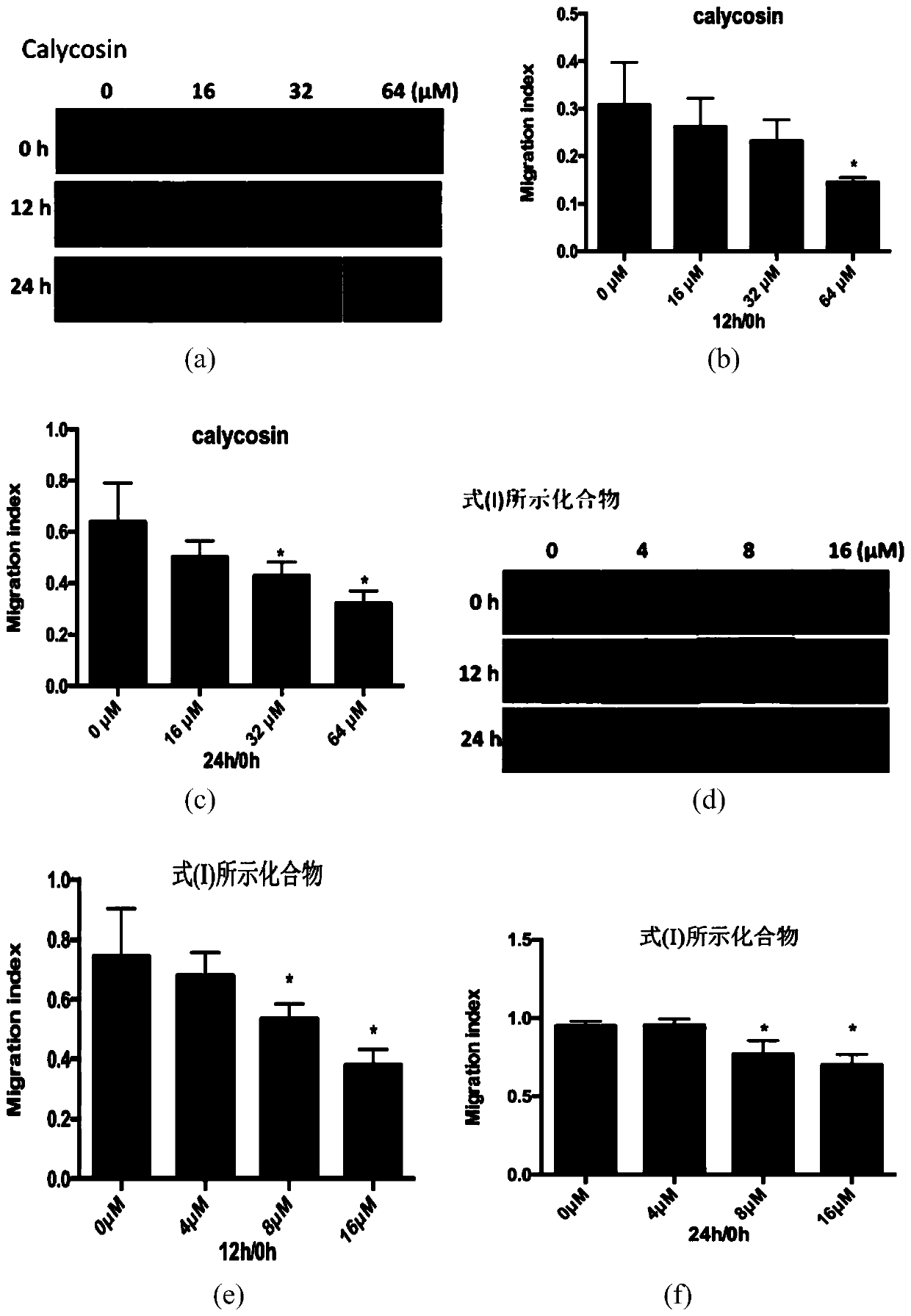
[0046] Experimental example 2: Plate cloning experiment
[0047] HUVEC was seeded in a 6-well plate at low density (500 cells / well in triplicate) and cultured for two weeks. Then, the cells were washed twice with PBS, fixed with 4% paraformaldehyde for 15 minutes, and stained with Gram staining solution for 20 minutes. The experimental results showed that with the increase of the concentration of CAG002, the number of HUVECs cloned cells decreased significantly, and the effect was most obvious at 16μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com