Novel method for preparing drotaverine hydrochloride intermediate
A technology for drotaverine hydrochloride and intermediates, which is applied in the field of preparation of drotaverine hydrochloride intermediates, and can solve the problems of higher requirements for operators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
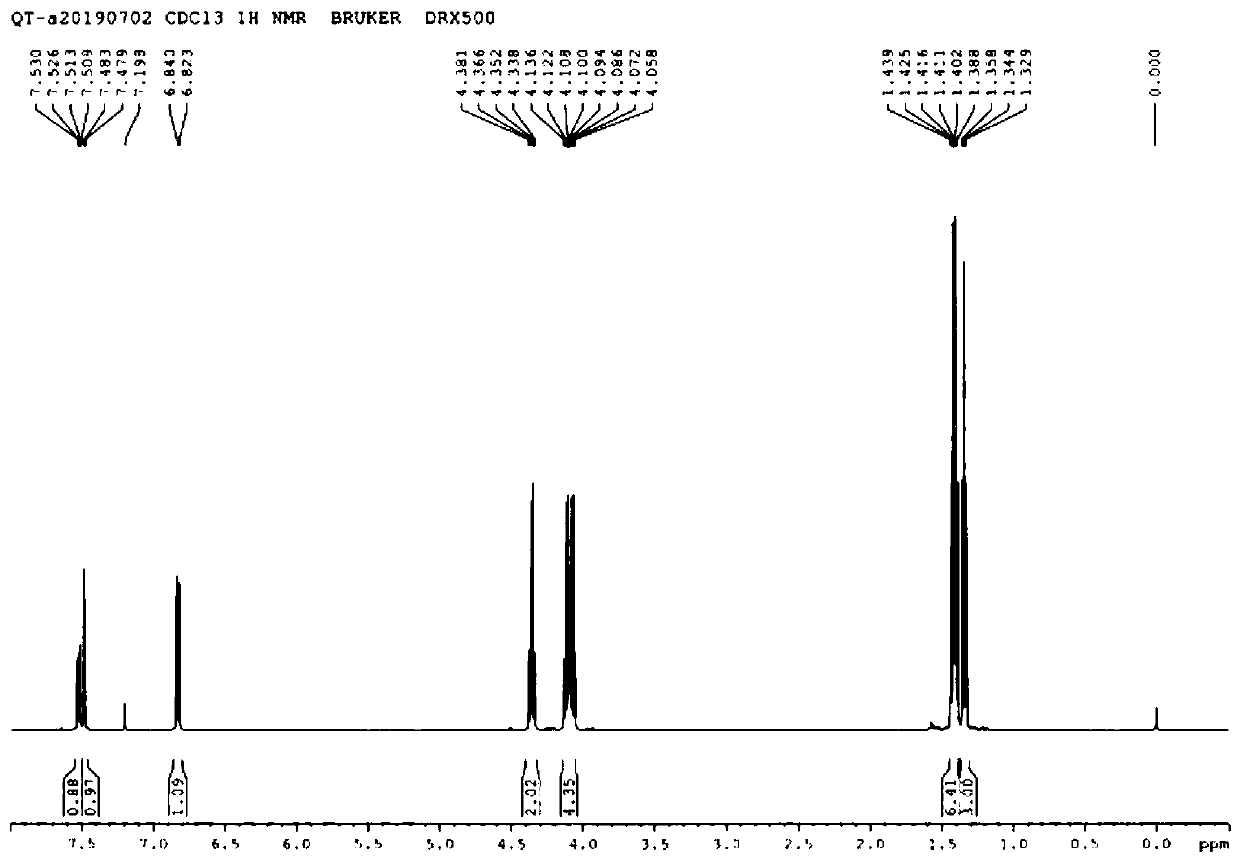
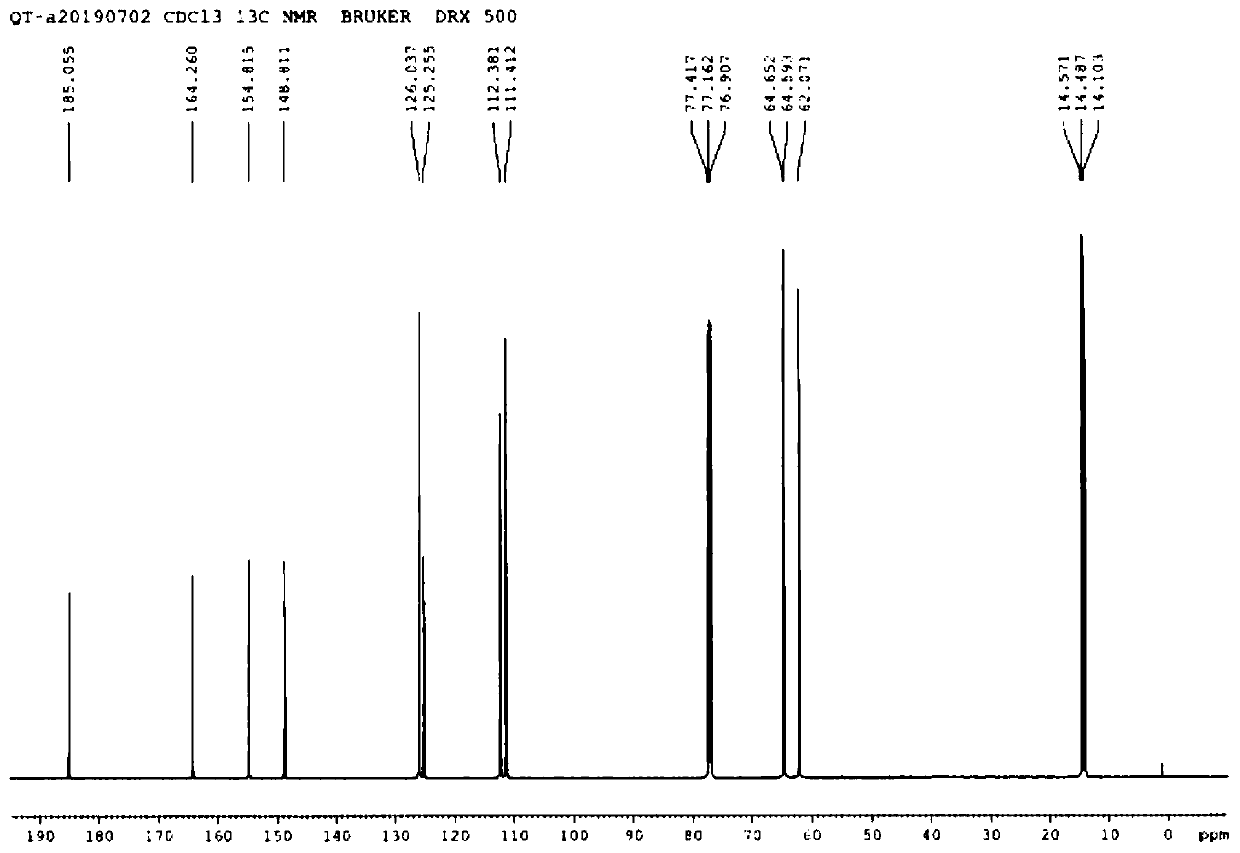
[0053] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenylacetic acid (I), reaction process is as follows:
[0054]
[0055] Specifically include the following steps:
[0056] 1) Synthesis of 2-(3,4-diethoxy)phenyl-glyoxylic acid ethyl ester (IV)
[0057] Add 500mL dichloromethane, 260mmol AlCl 3 , stir. Cool down to -10~-5°C, and add 205 mmol ethyl oxalyl chloride dropwise. After stirring for 15 minutes, a solution of 200 mmol of 1,2-diethoxybenzene (III) in dichloromethane (60 mL) was added dropwise. After the dropwise addition, the temperature was raised to room temperature, and the reaction was completed for 1 hour. The temperature was lowered to below 0°C, and 150 mL of cold water was slowly added dropwise to the reaction solution. Transfer to a separatory funnel and let stand to separate layers. After the water layer was separated, it was washed once with 150 mL of saturated sodium bicarbonate solution, then once with 15...
Embodiment 2
[0065] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenylacetic acid (I), reaction process is as follows:
[0066]
[0067] Specifically include the following steps:
[0068] 1) Synthesis of 1-(3,4-diethoxyphenyl)ethanone (VI)
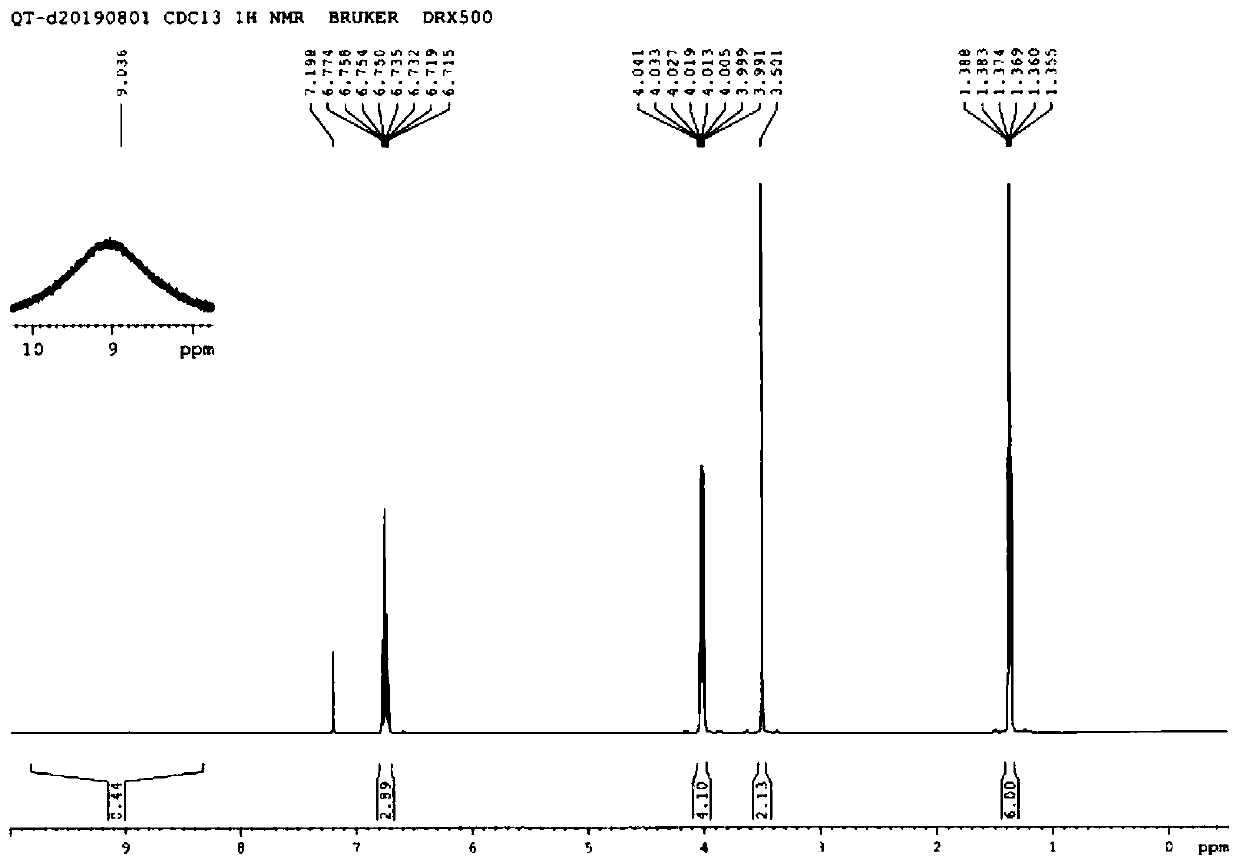
[0069] Add 240 mL of dichloromethane, 338 mmol of zinc chloride, and 242 mmol of acetyl chloride into the reaction flask and stir. After cooling to below 0°C, add 241 mmol of 1,2-diethoxybenzene (III) in 80 mL of dichloromethane dropwise, and react at room temperature for 1 hour after the dropwise addition. Cool to below 0°C, add 100 mL of water and stir for 15 minutes. Transfer to a separatory funnel to separate the layers. The dichloromethane layer was washed with 50 mL of 2N sodium hydroxide solution, then washed with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a light yellow oil, which solidified into an off-white solid after refrigeration to obtain 2...
Embodiment 3
[0074] A kind of preparation new method of drotaverine hydrochloride intermediate 3,4-diethoxyphenethylamine (II), reaction process is as follows:
[0075]
[0076] Specifically include the following steps:
[0077] 1) 3, the synthesis of 4-diethoxyphenylacetamide (VII)
[0078] Add 134 mmol of 3,4-diethoxyphenylacetic acid (I), 150 mL of dichloromethane, and 0.5 mL of DMF into the reaction flask, and stir. Below 10°C, 161 mmol of oxalyl chloride was added dropwise, and then heated to room temperature to react for 1 hour. Dichloromethane was removed by concentration under reduced pressure. The residue was dissolved in 200 mL of tetrahydrofuran and transferred to a reaction flask. 100 mL of 50% aqueous hydroxylamine solution was added dropwise at 40°C and reacted at 40-50°C for 1 hour. Concentrate under reduced pressure to remove tetrahydrofuran, and adjust the pH value to 7-8 with 2N hydrochloric acid. Extract with ethyl acetate 100mL. The organic layer was washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com