Selenized/sulfurized sulfuryl arylamine compound and selenylation/sulfurization method thereof
A technology for sulfurizing sulfonyl arylamine and sulfonyl arylamine, which is applied in the fields of sulfide preparation, organic chemistry, antibacterial drugs, etc., can solve the problems of complex reaction system, cumbersome post-processing, long reaction time, etc., and achieves easy preparation conditions. The effect of obtaining, wide derivation and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
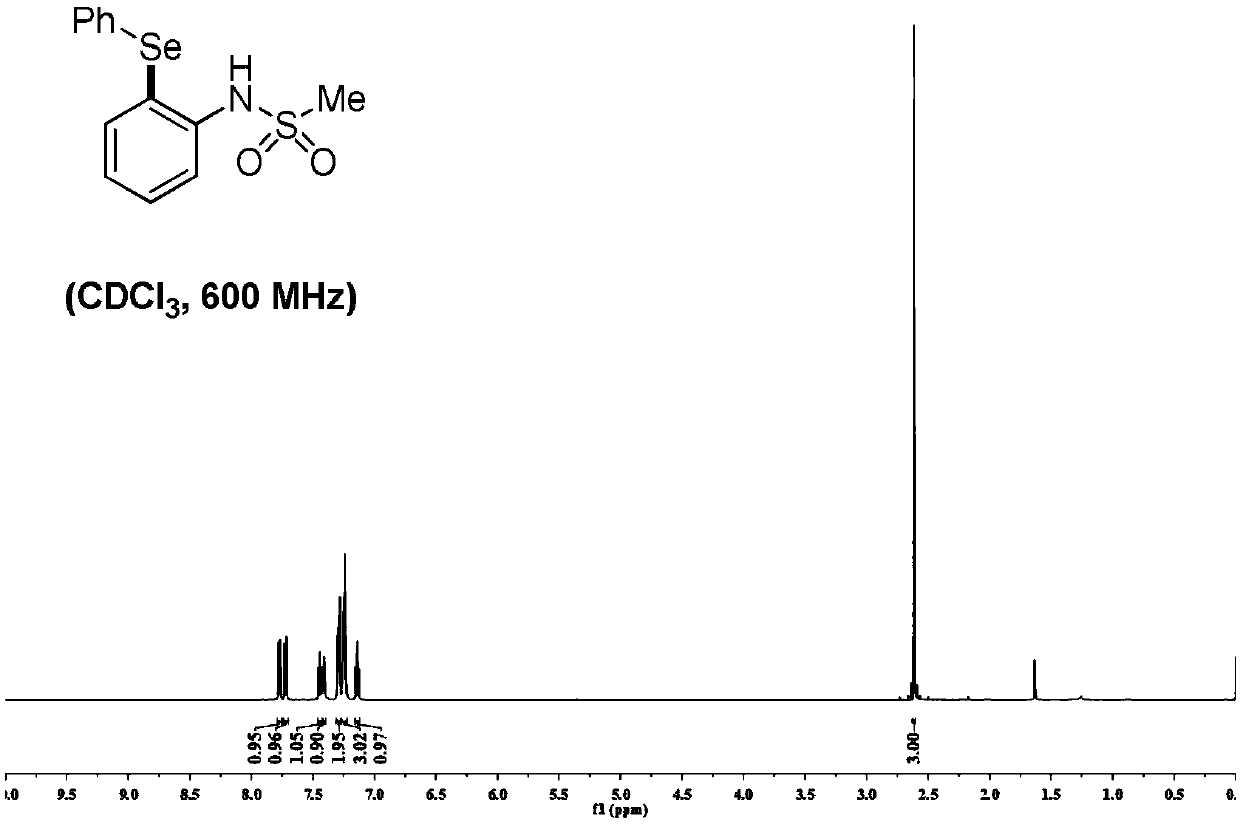
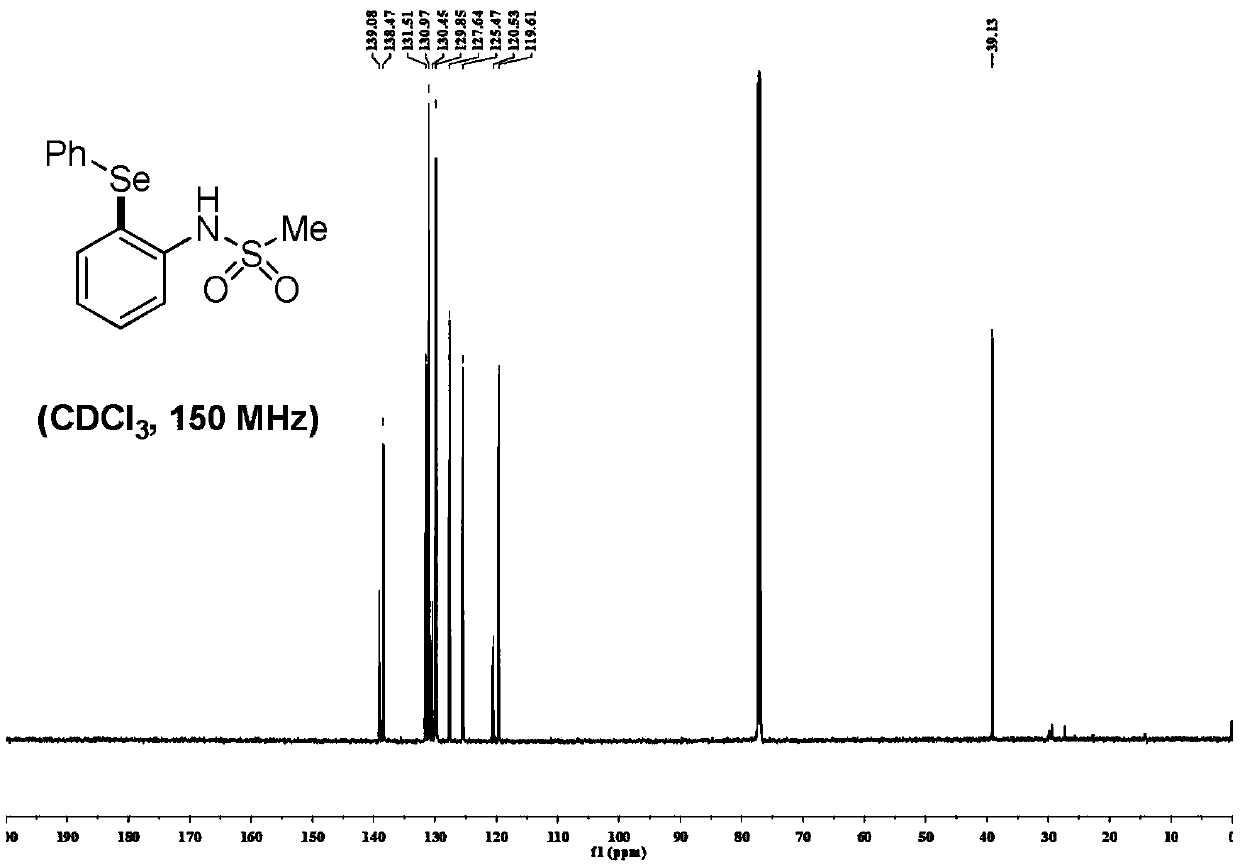
[0048] Mix 0.25 mmol of sulfonyl arylamine substrate 1 and 2 times the molar amount of diaryl diselenide 2, and add catalyst Pd(TFA) in a molar amount of 10% of the molar amount of the sulfonyl aryl amine substrate 2 and oxidant Cu(OAc) of 2 times the molar amount of sulfonyl arylamine substrate 2 Add 3 mL of dry toluene to the reaction tube under the protection of argon, and react at 125°C for 12 hours to obtain a mixed liquid. After the reaction, add water and ethyl acetate to extract three times, combine the organic layers, and anhydrous Na 2 SO 4 The organic layer was dried, filtered, concentrated, separated and purified by silica gel column chromatography (mobile phase: petroleum ether / ethyl acetate=30 / 1-5 / 1) to obtain selenide sulfonyl arylamine compound 3.
[0049]
[0050] R 1 for H, -CH 3 , -Cl, -F, -Br, -CF 3 ,-Et,-OCH 3 , 1,3 dioxane, 1,4 dioxane, benzene ring, one of bromobenzene, R 1 is a para and / or meta substitution;
[0051] When it is 1,3-dioxane or ...
Embodiment 2
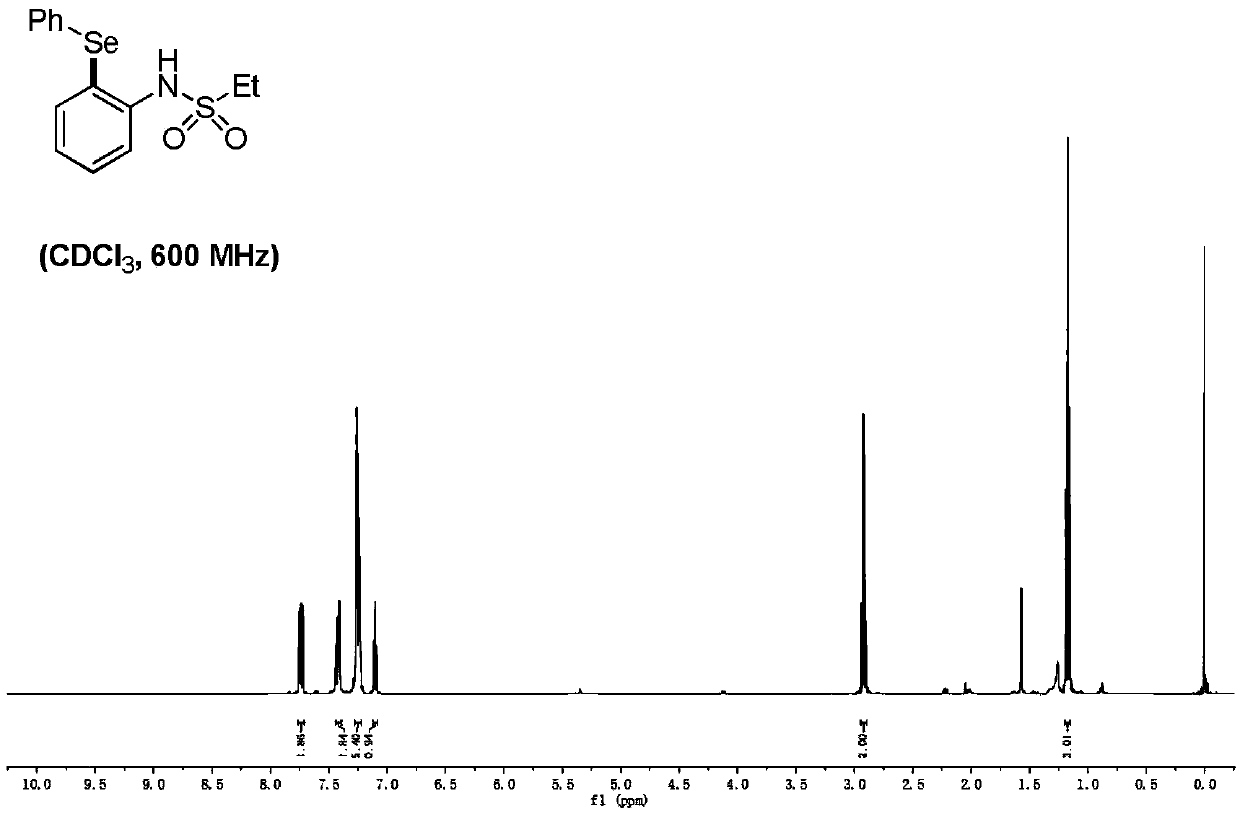
[0062] Add the catalyst Pd (TFA) of 10% molar quantity of sulfonyl arylamine substrate molar quantity 2 and oxidant Cu(OAc) of 2 times the molar amount of sulfonyl arylamine substrate 2 Add 3 mL of dry toluene to the reaction tube under the protection of argon, and react at 125°C for 12 hours to obtain a mixed liquid. After the reaction, add water and ethyl acetate to extract three times, combine the organic layers, and anhydrous Na 2 SO 4 The organic layer was dried, filtered, concentrated, separated and purified by silica gel column chromatography (mobile phase: petroleum ether / ethyl acetate=30 / 1-5 / 1) to obtain sulfonyl arylamine compound 5.
[0063]
[0064] R 1 for -CH 3 , -Cl, -CF 3 ,-Et,-OCH 3 , 1,3 dioxane;
[0065] R 2 for -CH 3 ;
[0066] R 3 is -Ph(phenyl), One of them, where R 4 It is one of -Cl, methyl, and methoxy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com