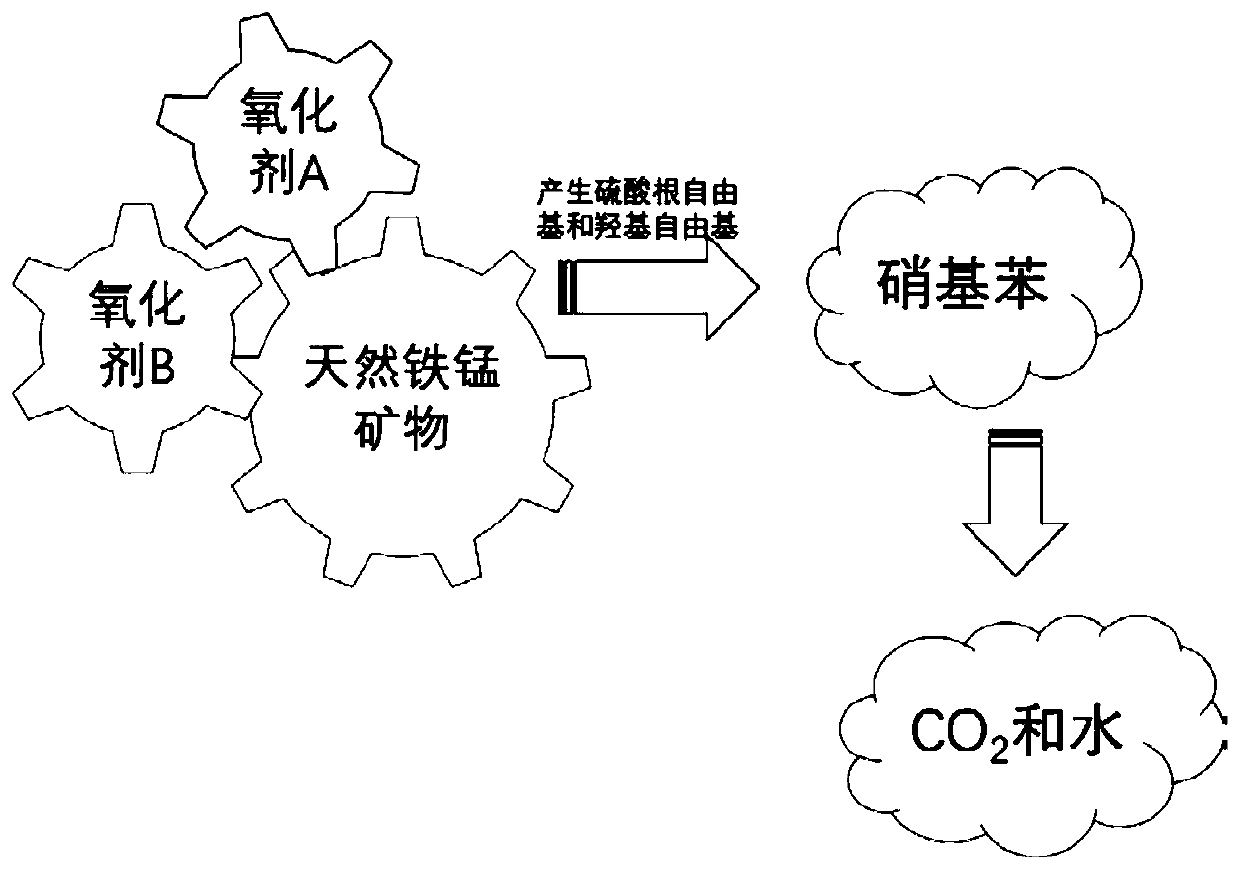
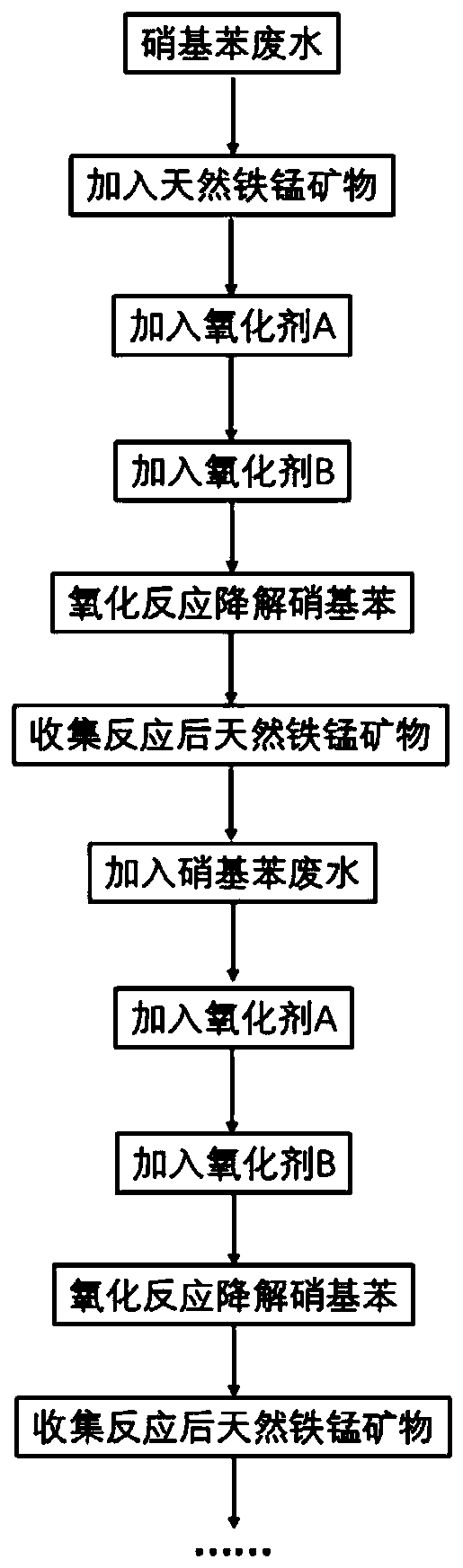
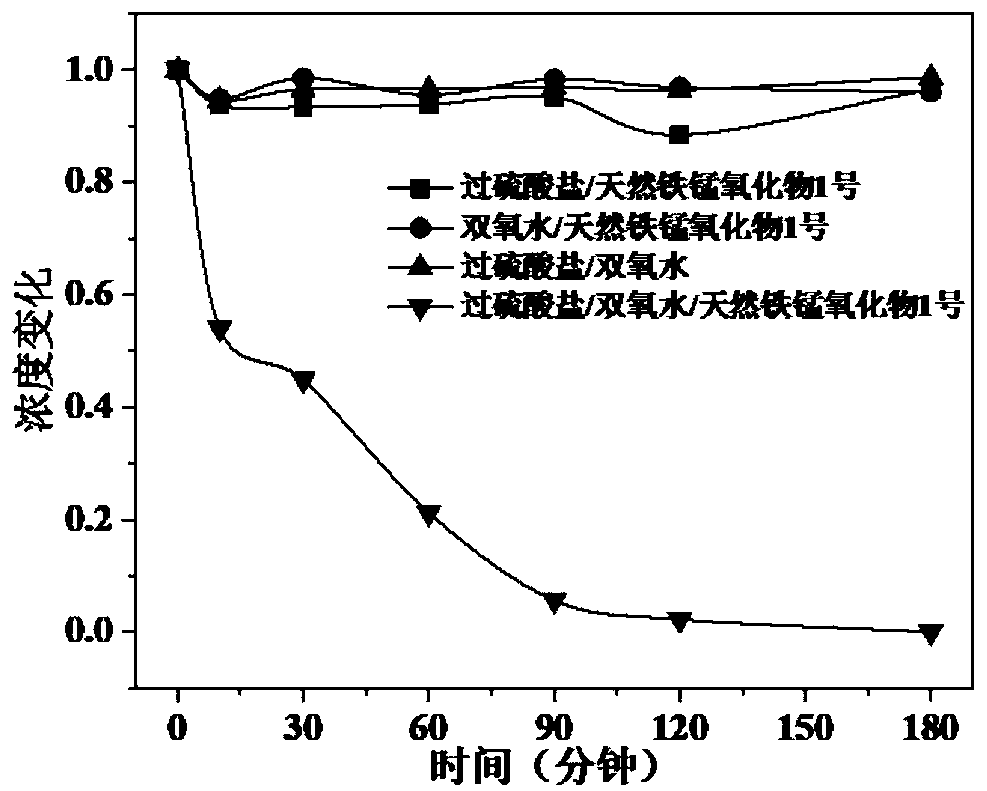
Method for degrading nitrobenzene wastewater by catalyzing double oxidants through natural ferromanganese mineral
A nitrobenzene wastewater, double oxidant technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low efficiency and achieve low iron and manganese The amount of exudation, abundant sources, and the effect of promoting chain reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The natural ferromanganese ore selected in this embodiment is used as a catalyst, and the oxidant A is hydrogen peroxide, and the oxidant B is sodium persulfate, and the catalytic oxidant A and the oxidant B degrade 2,4-dinitrotoluene with different initial concentrations, and the specific steps are as follows:
[0042] (1) Take a 120mL Erlenmeyer flask as a reactor, configure a 2,4-dinitrotoluene solution with an initial concentration of 5mg / L, select a reaction volume of 100mL, and set an initial pH of 7.13.
[0043] (2) Select No. 1 natural iron-manganese mineral. After XRF characterization, it is known that the iron oxide is 51.35%, the manganese oxide is 19.69%, and also contains other impurities. See Table 1 for details. The particle size is selected as 100 mesh. The addition amount was set to 1g / L, added to the reactor in step (1), the stirring was set at 250rpm, and the reaction was carried out for 10min.
[0044] (3) Add oxidant A to the reactor, choose to be h...
Embodiment 2
[0048] The natural ferromanganese ore selected in this embodiment is used as a catalyst, the oxidant A is hydrogen peroxide, and the oxidant B is potassium peroxomonosulfate, and the catalytic oxidant A and B degrade 2,4-dinitrotoluene with different initial concentrations, and the specific steps are as follows:
[0049] (1) Take a 120mL Erlenmeyer flask as a reactor, configure 2,4-dinitrotoluene solutions with an initial concentration of 5mg / L, 10mg / L, 15mg / L and 20mg / L, and select 100mL for the reaction volume. For 5mg / L 2,4-dinitrotoluene solution, adjust the pH to 3.03, 5.02, 7.03, 9.02 and 10.99.
[0050] (2) Select No. 2 natural iron-manganese minerals. After XRF characterization, it is known that iron oxides are 52.41%, manganese oxides are 10.93%, and other impurities are also contained. See Table 2 for details. The particle size is selected to be 100 mesh. Adding amount is set to 1g / L, add to the reactor in step (1), stir at 300rpm, and react for 30min.
[0051] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com