Fusion gene MBP-H1 expressing heparinase, recombinant plasmid of fusion gene MBP-H1 and application of recombinant plasmid
A technology of MBP-H1 and fusion gene, which is applied in the fusion gene MBP-H1 expressing heparanase and its recombinant plasmid and application field, which can solve the problems of high cost of heparanase preparation and limited source of heparanase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
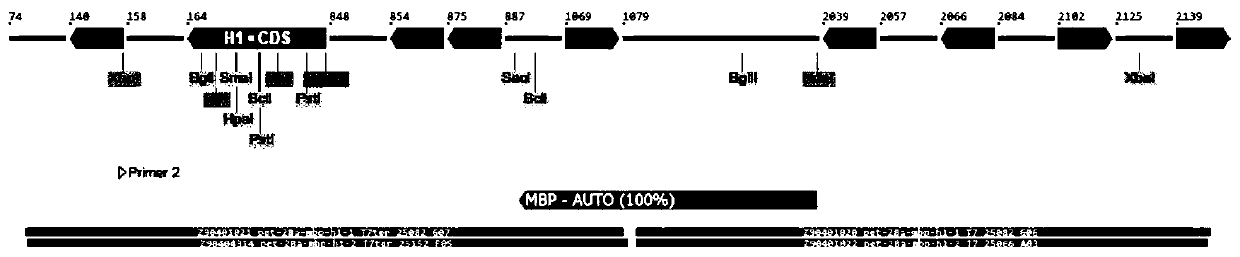
[0056] 1.11 Entrust Sangon Bioengineering (Shanghai) Co., Ltd. to synthesize the MBP-H1 sequence (as shown in SEQ ID NO. 1).
[0057] 1.12 Culture the strain E.coli TOP10 pET-28a, extract the plasmid and double-enzyme digestion The above-purified PCR product and the vector plasmid pET-28a were double-enzyme digestion with XbaI and XhoI restriction endonucleases, and the double-enzyme digestion conditions were: : 37°C digestion for 2h, the double digestion reaction system (50μL) is shown in Table 4:
[0058] Table 4. Composition of double-enzyme digestion system
[0059]
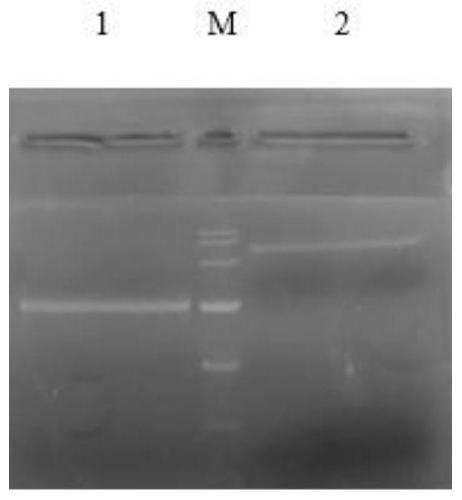
[0060] Agarose gel electrophoresis was performed on the enzyme-digested product of the above-mentioned target gene and the vector plasmid, followed by gel cutting and recovery. The result is as figure 1 As shown, lane M is DL10000 DNAMarker; lane 1 is the double-enzyme digestion product of the target gene; lane 2 is the plasmid double-enzyme digestion product; the target gene and plasmid band after doubl...
Embodiment 2
[0084] 2.1 Experimental method
[0085] 2.1.1 Optimization of IPTG concentration of inducer
[0086] Take 50 μL of E.coli BL21(DE3)pET-28a-MBP-H1-2 stored in a glycerol tube, put it into 5 mL of LB medium containing 50 μg / mL kanamycin, and culture at 37°C, 200 rpm, overnight. Take 100 μL of seed solution and add it to 10 mL of LB medium (containing 50 μg / mL kanamycin), cultivate at 37 ° C and 200 rpm until the OD600 is about 0.7, and add IPTG with final concentrations of 0.1, 0.25, 0.5, 0.75, 1mM, each group. Do three parallel controls, 37 ℃, 200rpm, induction 10h. Add 200 μL / well to 96-well plate, measure OD with a microplate reader 600 , and then crushed the cells to extract the crude enzyme solution to measure the enzyme activity.
[0087] Induction results such as Figure 4 shown, where -●- represents OD 600 ;-■- represents enzyme activity (U / L); with the increase of IPTG concentration, the general direction of the enzyme activity curve is a gradual decrease, and its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com