Medical composition having maltose hydrolase inhibitory activity, and application of medical composition
A technology for inhibiting activity and maltase, applied in the field of medicine and biology, can solve the problem of large side effects of maltohydrolase inhibitors, and achieve the effects of improving drug efficacy, reducing postprandial blood sugar, and solving hypoglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
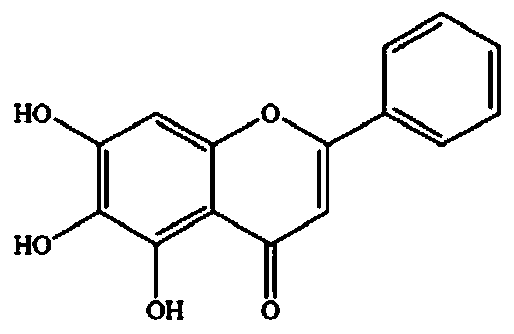
[0056] Example 1 Synergistic inhibition of baicalein and 1-DNJ on maltose hydrolase in the small intestine of mice
[0057] Kunming mice (20~23g) were fasted for 16 hours, and the small intestines were taken after the neck was severed, cut open, washed with pre-cooled PBS (0.1M, pH7.0) buffer, and then according to 1:10 (W / V) Add PBS buffer. The small intestine was cut into fragments and homogenized, centrifuged at 4°C, 10000r / min, for 15min, and the supernatant was taken as the mother liquor of α-glucosidase for the test.
[0058] The total volume of the enzyme reaction system is 250 μL, including 50 μL of enzyme solution, baicalein, 1-DNJ, maltose, and buffer solution, in which baicalein is dissolved in 50% DMSO. First, mix the enzyme with the two inhibitors, incubate at 37°C for 30 minutes, and add 50 μL of maltose solution to start the reaction. Incubate at 37°C and react for 20 minutes. The sample was placed in boiling water for 5 minutes to stop the reaction. Use the gluc...
Embodiment 2
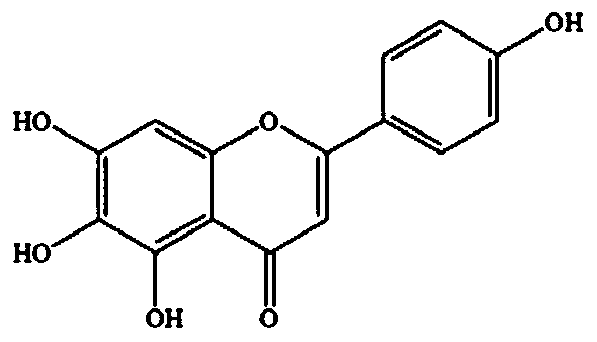
[0062] Example 2 Synergistic inhibition of wild scutellarin and 1-DNJ on maltose hydrolase in the small intestine of mice
[0063] The method for the preparation and inhibition rate determination of α-glucosidase is similar to that of Example 1, except that the flavonoids are replaced with scutellarin. Combining 21.5, 43, 86, 172, 344 μM scutellaria with 0.023, 0.046, 0.092, 0.184, 0.368 μM 1-DNJ, respectively, the inhibition rate of the combined use was significantly higher than that of single use at each dose point ( Table 2), CI values are all less than 0.6, and some representative CI values of synergistic inhibition are as follows image 3 As shown, the results show that the combined use of scutellarin and 1-DNJ has a strong synergistic inhibitory effect on the process of maltose hydrolase hydrolyzing maltose.
[0064] Table 2. The CI index of scutellarin and 1-DNJ synergistically inhibit the intestinal maltose hydrolase of mice
[0065]
[0066]
Embodiment 3 6
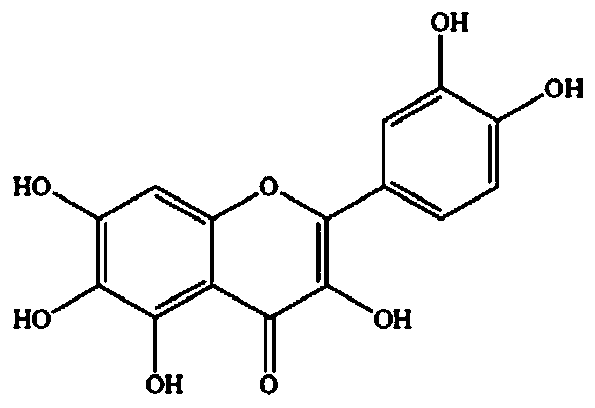
[0067] Example 3 Synergistic inhibition of hexahydroflavonoids and 1-DNJ on maltose hydrolase in the small intestine of mice
[0068] The method for the preparation of α-glucosidase and the determination of inhibition rate is similar to that of Example 1, except that the flavonoids are replaced with hexahydroflavonoids. Combining 25, 50, 100, and 200 μM hexahydroxyflavonoids with 0.023, 0.046, 0.092, 0.184 μM 1-DNJ, respectively, the inhibition rate of the combined use was significantly higher than that of the single use at each dose point (Table 3), CI values are all less than 0.8, and some representative CI values of synergistic inhibition are such as Figure 4 As shown, the results indicate that the combined use of hexahydroxyflavone and 1-DNJ has a strong synergistic inhibitory effect on the process of maltose hydrolase hydrolyzing maltose.
[0069] Table 3. The CI index of hexahydroflavonoids and 1-DNJ synergistically inhibiting maltose hydrolase in the mouse intestine
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com