Anti-novel coronavirus naphthoquinone compound and medical application thereof
A technology of coronaviruses and compounds, applied in the field of medicine, to achieve good prospects for new drug development, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
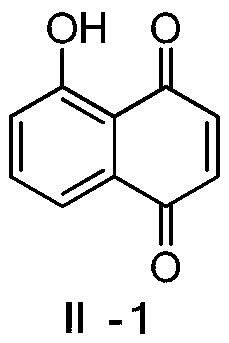
[0024] This embodiment relates to a preparation method of 5-hydroxy-1,4-naphthoquinone (II-1) having structural formula (II), comprising the following steps:
[0025]
[0026] Add 75mL 30% H to a 250mL three-necked bottle 2 o 2 , stirred and added dropwise 42mL of acetic anhydride, and kept the temperature of the reaction solution not exceeding 40°C during the dropwise addition. After dropping, stir at 40°C for 4 hours. Afterwards, 100 mL methanol solution of 1,5-dihydroxynaphthalene (15 g, 93.7 mmol) was added dropwise to the reaction solution under vigorous stirring, and the rate of addition was controlled so that the temperature of the reaction solution did not exceed 60°C. Continue to stir for 1 hour after the dropwise addition, pour into ice water after cooling, a large amount of tan precipitate precipitates, filter and dry to obtain a dark red solid powder. Silica gel column chromatography (mobile phase: ethyl acetate-petroleum ether volume ratio: 1:5) was used for...
Embodiment 2
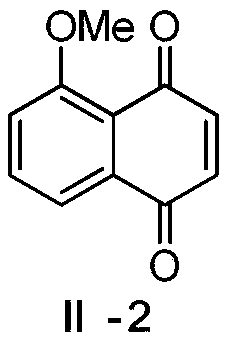
[0028] This embodiment relates to a preparation method of 5-methoxy-1,4-naphthoquinone (juglone methyl ether) (II-2) having structural formula (II), comprising the following steps:
[0029]
[0030] Compound II-1 (3g, 17.2mmol) described in Example 1 was dissolved in 90mL of THF-H 2 To the O (2:1) mixed solution, tetrabutylammonium bromide (1.6g, 5.2mmol), sodium hydroxide (11.2g, 64.3mmol) and sodium hydroxide (9.6g, 240mmol) were sequentially added under stirring. Gabby, in N 2Under protection, dimethyl sulfate (10 mL, 103 mmol) was added dropwise. After the dropwise addition was completed, react at room temperature for 4 h and post-treatment. The reaction solution was extracted with ethyl acetate (80 mL×3), and the organic layers were combined; the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (mobile phase: ethyl acetate-petroleu...
Embodiment 3
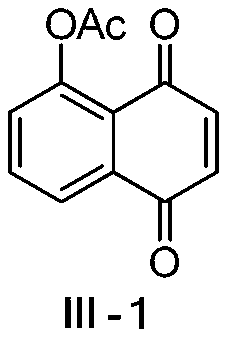
[0032] This embodiment relates to a preparation method of 5-acetoxy-1,4-naphthoquinone (5-acetyljuglone) (III-1) having the structural formula (III), comprising the following steps:
[0033]
[0034] Compound II-1 (3 g, 17.2 mmol) described in Example 1 was dissolved in 10 mL of acetic anhydride, and 5 drops of concentrated sulfuric acid were added dropwise with stirring. The reaction solution was placed in an ice-water bath and stirred for 2 hours. Afterwards, the reaction solution was suction-filtered, washed with ice water and a small amount of methanol successively to obtain about 3.4 g of compound III-1 in the form of light yellow powder crystals, and the yield was 91%. 1 H NMR (4 00MHz, CDCl 3 ): δ8.05(dd, J=7.9, 1.0Hz, 1H), 7.77(t, J=7.9Hz, 1H), 7.40(dd, J=7.9, 1.0Hz, 1H), 6.95(d, J= 10.3Hz,1H),6.85(d,J=10.3Hz,1H),2.45(s,3H,CH 3 CO).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com