Pyridine imine ligands, pyridine imine palladium complexes based on the pyridine imine ligands and catalytic application of the pyridine imine palladium complexes
A technology of pyridinimine palladium and pyridinimine, which is applied in the field of catalysts for α-olefin polymerization, can solve the problems of poor printability and dyeability of polymers, achieve good catalytic activity and improve performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The structural formula of the pyridine imine ligand prepared in this embodiment is shown in formula L1 or L2:
[0053]
[0054] The synthesis process is as follows: the amine compound (5mmol) shown by the structural formula as A1 or A2 is mixed with pyridine-2-carboxaldehyde (10mmol) in molar ratio, dissolved in 40mL of toluene, and then 30mg of p-toluenesulfonic acid (PTSA) is added The resulting solution was stirred and refluxed at 90°C for 24 h; after the resulting reaction solution was cooled to room temperature, part of the toluene was evaporated under reduced pressure until a pale yellow solid appeared, and then the evaporated reaction solution was diluted with 100 mL of methanol, at this time A large amount of light yellow solid precipitated; the resulting yellow solid was collected by filtration, and recrystallized from dichloromethane and hexane, the precipitate was collected by filtration, washed with methanol, and dried at room temperature to obtain the tar...
Embodiment 2
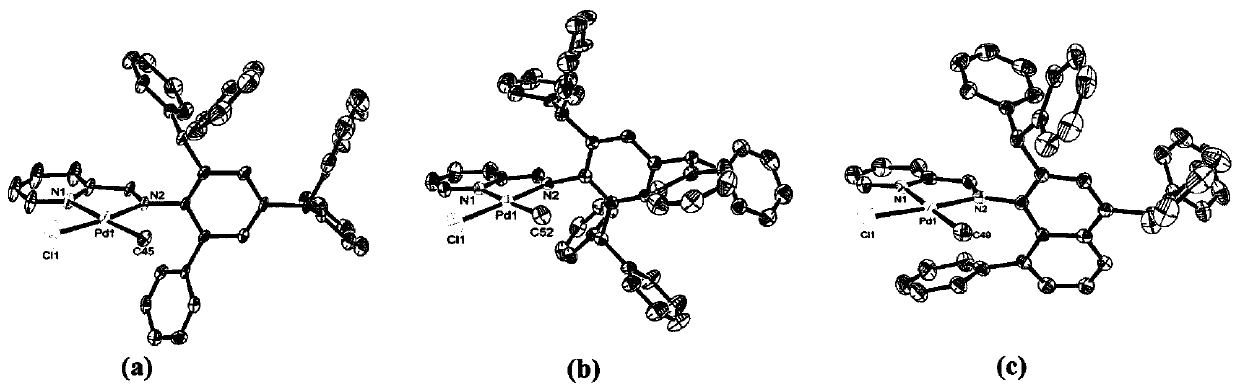
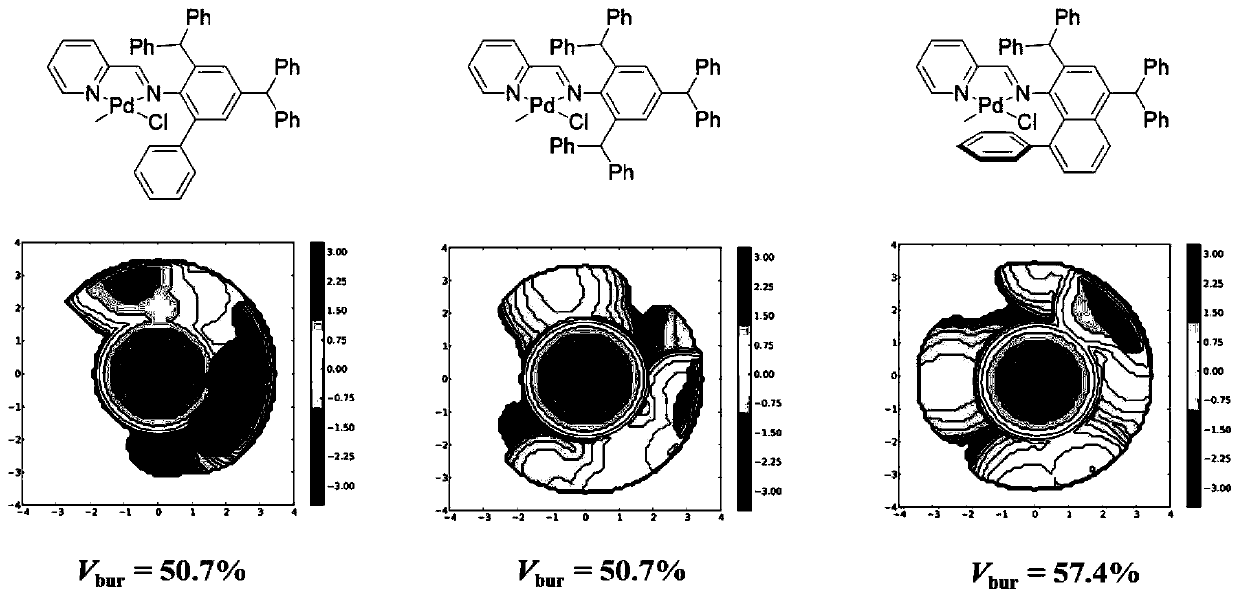
[0063] The present embodiment prepares pyridine imine palladium complex Pd1, Pd2, Pd3, and its structure is as follows:
[0064]
[0065] The synthesis process is as follows: dissolve 1mmol of pyridine imine ligand L1, L2 or L3 and (COD)PdMeCl (265mg, 1mmol) in 20mL of dichloromethane, stir and react at room temperature for 24h; during the stirring process, the color of the solution deepens . After the reaction was completed, most of the methylene chloride was evaporated under reduced pressure, the solution was concentrated to 5 mL, and 20 mL of diethyl ether was added to dilute the solution. At this time, a large amount of yellow solid precipitated out, which was filtered and washed with diethyl ether (3×5 mL). Promptly obtain the pyridinimine palladium complex, then dry it under reduced pressure at room temperature for about 5 hours, and save it for future use. The yield, nuclear magnetic analysis, and mass spectrometry analysis of the pyridinimine palladium complexes Pd1...
Embodiment 3
[0076] The general method for ethylene homopolymerization using pyridinimine palladium complexes as catalysts is as follows: first, dry a 350mL stainless steel pressure reactor connected to a high-pressure gas pipeline at 90°C in vacuum for at least 1 hour; then adjust the reactor to the required polymerization temperature (the set temperature in this embodiment is 30°C, 50°C). Add 38 mL of dichloromethane and the required amount of NaBArF (20 μmol in this example) to the reactor under a nitrogen atmosphere, and then inject 2 mL of CH containing the desired pyridinimine palladium complex catalyst via a syringe. 2 Cl 2 Inject into the polymerization system (in this embodiment, the amount of catalyst added is 10 μmol). With rapid stirring, the reactor was pressurized and maintained at 4 atm ethylene pressure. After 3 h, the pressure reactor was vented and the polymer was dried under vacuum overnight. Utilize above-mentioned embodiment gained Pd1, Pd2, Pd3 the performance of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com