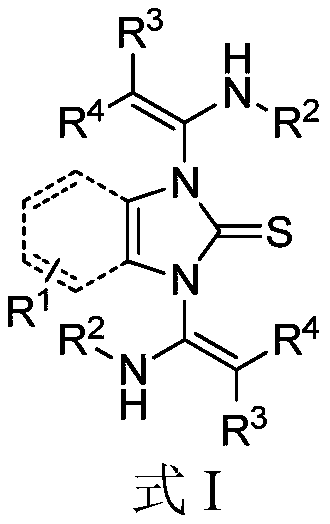
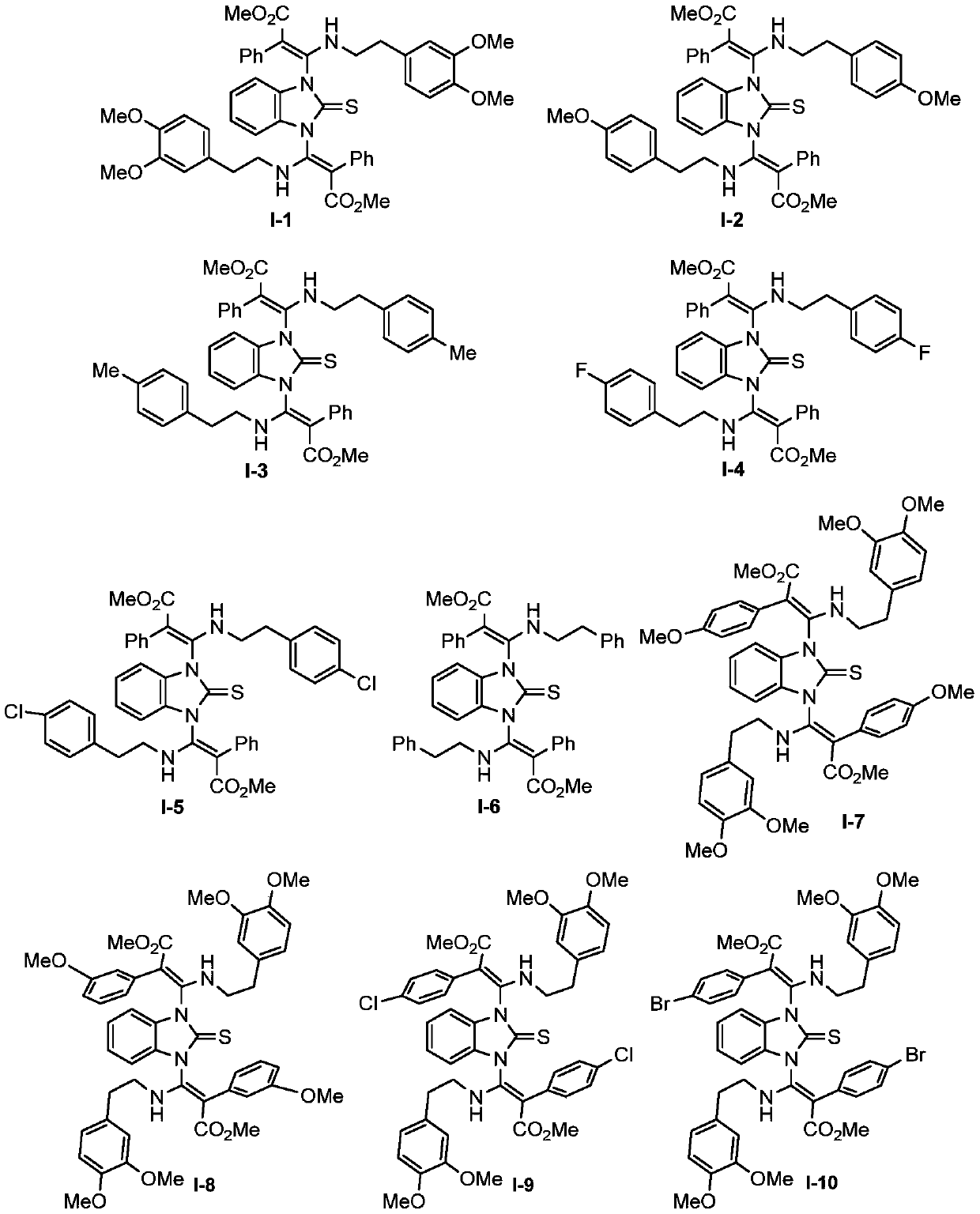
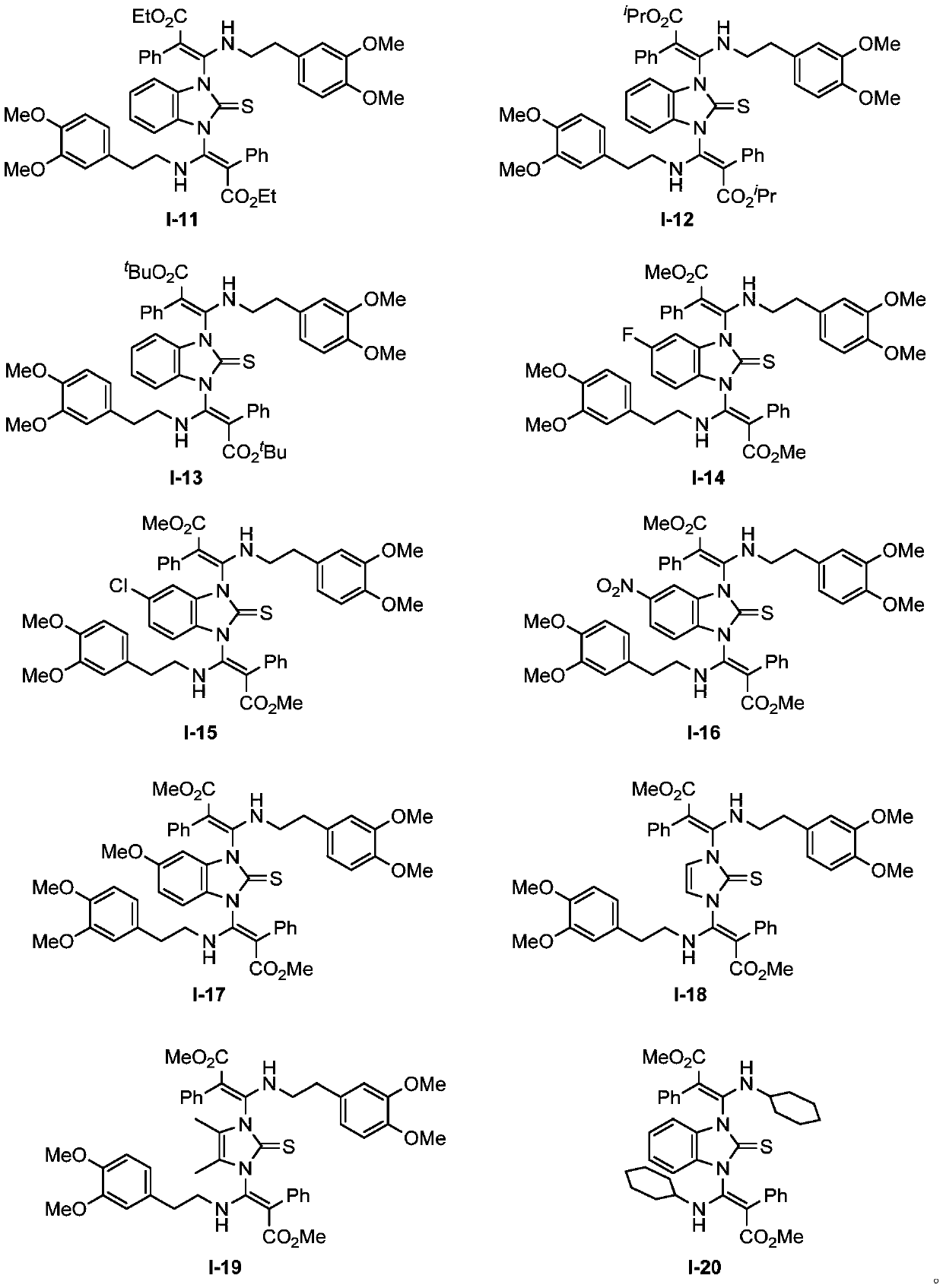
1,3-bis(beta-amino acrylate) substituted imidazole compound as well as preparation method and application thereof
A technology of amino acrylates and compounds, applied in organic chemistry, drug combinations, antibacterial drugs, etc., can solve the problems of multi-step synthesis operations and low atom utilization, and achieve good functional group compatibility, cheap and easy-to-obtain catalysts, The effect of excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 1,3-two (β-aminoacrylate) substituted imidazole compounds shown in formula I-1:
[0035]
[0036] Under nitrogen protection, 3-((3,4-dimethoxyphenethyl)imino)-2-phenylacrylate methyl ester Ⅱ-1 (101.7mg, 0.30mmol), 2- Mercaptobenzimidazole III-1 (7.5mg, 0.05mmol), catalyst [DABCO] (1.7mg, 0.015mmol) and solvent CH 3 CN (1.0 mL). After the reaction solution was stirred at 70° C. for 1 h, TLC detected that the raw materials were basically reacted, and the reaction was stopped. The reaction solution was subjected to direct column chromatography with eluent (petroleum ether / ethyl acetate=5 / 1) to obtain 38.1 mg of product I-1 as a white solid with a yield of 92%.
[0037] The analytical data of 1,3-bis(β-aminoacrylate) substituted imidazole compounds shown in formula I-1: 1H NMR (500MHz, CDCl 3):δ2.11-2.18(m,2H),2.58-2.67(m,4H),3.07-3.12(m,2H),3.632(s,6H),3.634(s,6H),3.88(s,6H ),6.35-6.39(m,4H),6.58(dd,J=8.5,2.0Hz,2H),6.75(d,J=8.0Hz,2H),6.83-6.90(m,8H),7.0...
Embodiment 2
[0039] Synthesis of 1,3-two (β-aminoacrylate) substituted imidazole compounds shown in formula I-2:
[0040]
[0041] Under nitrogen protection, 3-((4-methoxyphenethyl)imino)-2-methyl phenylacrylate II-2 (92.7mg, 0.30mmol), 2-mercaptobenzo Imidazole III-1 (7.5mg, 0.05mmol), catalyst [DABCO] (3.4mg, 0.03mmol) and solvent CH 3 CN (5.0 mL). After the reaction solution was stirred at 50° C. for 5 h, TLC detected that the raw materials had basically reacted, and the reaction was stopped. The reaction solution was subjected to direct column chromatography, eluent (petroleum ether / ethyl acetate=5 / 1), and 36.9 mg of white solid of product I-2 was obtained, with a yield of 96%.
[0042] The analytical data of 1,3-bis(β-aminoacrylate) substituted imidazole compounds shown in formula I-2: 1 H NMR (400MHz, CDCl 3 ):δ2.13-2.23(m,2H),2.62-2.66(m,4H),2.98-3.05(m,2H),3.63(s,3H),3.64(s,3H),3.80(s,3H ),3.81(s,3H),6.59-6.63(m,2H),6.77-6.81(m,10H),6.92-6.97(m,6H),7.06(brs,4H),8.93-8.95(m,...
Embodiment 3
[0044] Synthesis of 1,3-two (β-aminoacrylate) substituted imidazole compounds shown in formula I-3:
[0045]
[0046] Under nitrogen protection, 3-((4-methylphenethyl)imino)-2-methyl phenylacrylate II-3 (73.3mg, 0.25mmol), 2-mercaptobenzimidazole Ⅲ-1 (7.5mg, 0.05mmol), catalyst [Et 3 N] (1.0 mg, 0.01 mmol) and the solvent DMF (0.5 mL). After the reaction solution was stirred at 80° C. for 2.5 h, TLC detected that the raw materials were basically reacted, and the reaction was stopped. The reaction solution was subjected to direct column chromatography with eluent (petroleum ether / ethyl acetate=5 / 1) to obtain 34.6 mg of product I-3 as a white solid with a yield of 94%.
[0047] The analytical data of 1,3-bis(β-aminoacrylate) substituted imidazole compounds shown in formula I-3: 1 H NMR (400MHz, CDCl 3 ): δ2.15-2.24(m,2H),2.34(s,6H),2.65(t,J=6.0Hz,4H),2.99-3.06(m,2H),3.63(s,6H),6.60- 6.62(m,2H),6.75-6.76(m,6H),6.92-6.94(m,6H),7.05-7.07(m,8H),8.93-8.95(m,2H); 13 C NMR (10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com