Preparation and purification method of olmesartan medoxomil key intermediate
A technology for olmesartan medoxomil and a purification method, applied in the field of chemical synthesis, can solve the problems of difficult separation and removal of by-products, difficult to realize industrialized production, poor product quality, etc., and achieves easy control of process conditions, simple post-processing and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
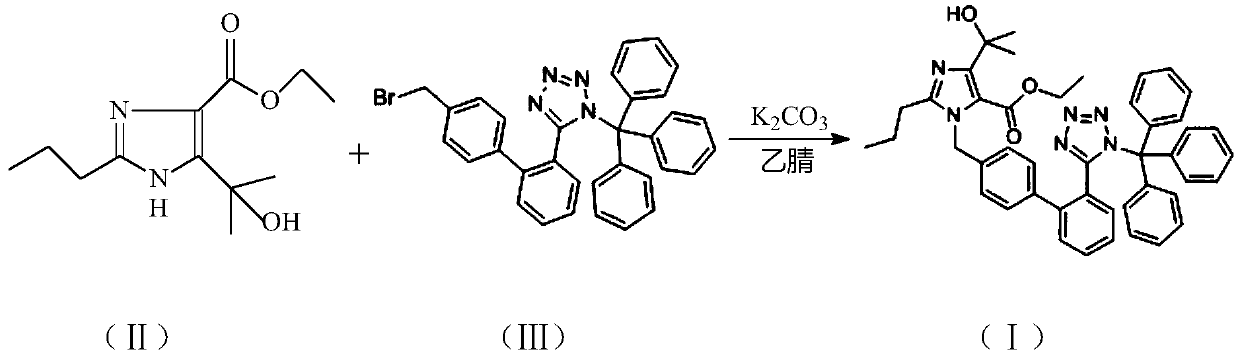
[0014] A. Add 660kg acetonitrile in the 1000L glass-lined reaction kettle equipped with devices such as agitator, thermometer vacuum pressure gauge, etc., add 30.0kg compound (II), 84kg compound (III) and 34.8kg potassium hydroxide powder under stirring, close the feeding After nitrogen replacement, keep the nitrogen positive pressure, the temperature is raised to 90 ℃ under stirring, the reaction process control pressure is ≥0.01Mpa≤0.3Mpa, and the temperature is maintained and stirred for 12 hours. After the reaction finishes, the temperature in the reaction mixture is lowered to 45~50 ℃, the insoluble by-product is removed by pressure filtration, the filtrate is concentrated under reduced pressure to recover the solvent to 3 / 4 of the added amount, the concentrated solution is cooled for crystallization, and the crystallization is incubated at 3 ℃ for 1 hour, Centrifuge to obtain the wet crude product of compound (I).
[0015] B, add 180kg of acetonitrile to the 500L refinin...
Embodiment 2
[0017] A. Add 600kg acetonitrile in the 1000L glass-lined reactor equipped with devices such as agitator, thermometer vacuum pressure gauge, etc., add 30.0kg compound (II), 76.6kg compound (III) and 30.2kg potassium hydroxide powder under stirring, close At the feeding port, after nitrogen replacement, keep nitrogen positive pressure, heat up to 85°C under stirring, control pressure ≥0.01Mpa≤0.3Mpa in the reaction process, keep stirring for 14 hours. After the reaction finishes, the temperature in the reaction mixture is lowered to 45~50 ℃, the insoluble by-product is removed by pressure filtration, the filtrate is concentrated under reduced pressure to recover the solvent to 3 / 4 of the added amount, the concentrated solution is cooled for crystallization, and the crystallization is incubated at 5 ℃ for 1 hour, Centrifuge to obtain the wet crude product of compound (I).
[0018] B, add 150kg of acetonitrile to the 500L refining kettle, start stirring, add the wet crude product...
Embodiment 3
[0020] A. Add 750kg of acetonitrile in the 1000L glass-lined reactor equipped with devices such as agitator, thermometer vacuum pressure gauge, add 30kg of compound (II), 84kg of compound (III) and 34.8kg of potassium hydroxide powder under stirring, close the feeding port , after nitrogen replacement, keep nitrogen positive pressure, heat up to 80 ℃ under stirring, control pressure ≥0.01Mpa≤0.3Mpa in the reaction process, keep stirring for 15 hours. After the reaction finishes, the temperature in the reaction mixture is reduced to 45~50 ℃, the insoluble by-product is removed by pressure filtration, the filtrate is concentrated under reduced pressure to recover the solvent to 3 / 4 of the added amount, the concentrated solution is cooled for crystallization, and the crystallization is incubated at 0 ℃ for 1 hour, Centrifuge to obtain the wet crude product of compound (I).
[0021] B, add 165kg of acetonitrile to the 500L refining kettle, turn on stirring, add the wet crude produ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com