TIM-3 nanobody as well as preparation method and application thereof
A technology of TIM-3 and nano-antibody, applied in biochemical equipment and methods, antibody, DNA preparation, etc., can solve the problems of high storage cost, high price of monoclonal antibody drugs, difficulty in large-scale production of monoclonal antibody, etc. achieve high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The vector construction method provided by the embodiment of the present invention is:
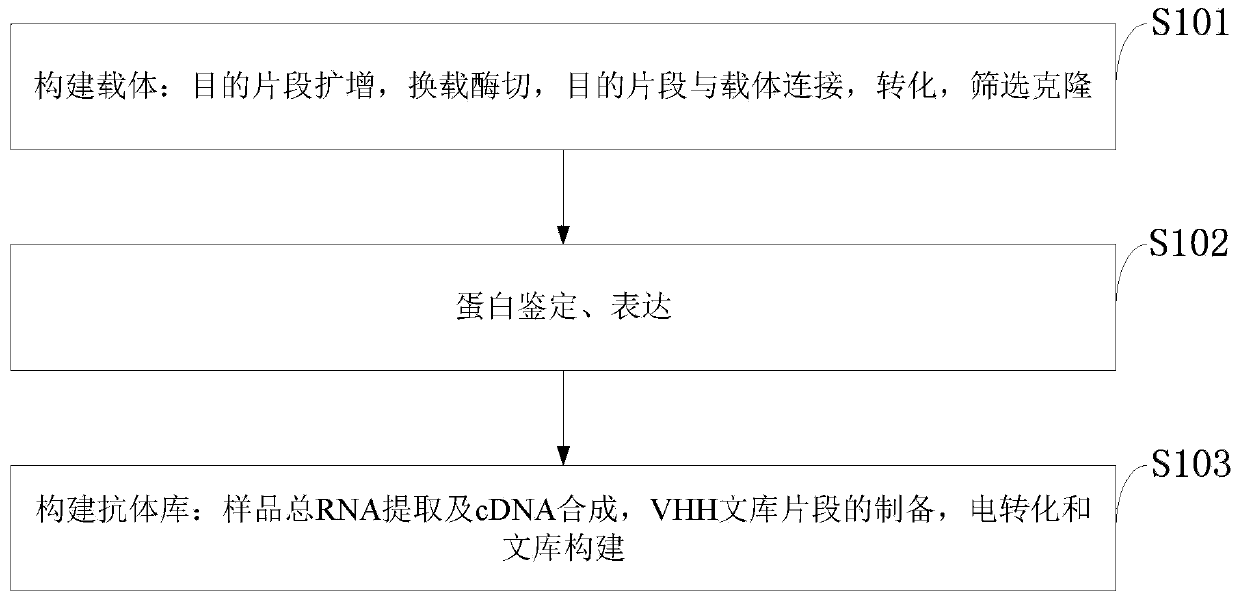
[0116] 1. Target fragment amplification is specifically:
[0117] (1) Design synthetic primers to amplify a sufficient amount of the target product by PCR.
[0118] (2) The PCR reaction uses pfu high-temperature polymerase.
[0119] The consumption of each component of PCR: (primer concentration is that 10D is dissolved in 400ul ddH 2 O)
[0120] Reaction system: 50ul. Primer mix, (1 / 28) 0.4ul x 13.6 total 8ul. 10X pfu Buffer, 5ul. Each upstream and downstream primers, each 2ul. Pfu, 0.4ul (5u / ul). wxya 2 O, replenish water to 50ul respectively.
[0121] The method amplification specific steps of target fragment PCR are:
[0122] (1) The first round of PCR procedure:
[0123]
[0124] The above is the first-round PCR reaction system, and the second-round PCR is performed with the first-round PCR product as a template.
[0125] (2) The second round of PCR reaction syst...
Embodiment 2
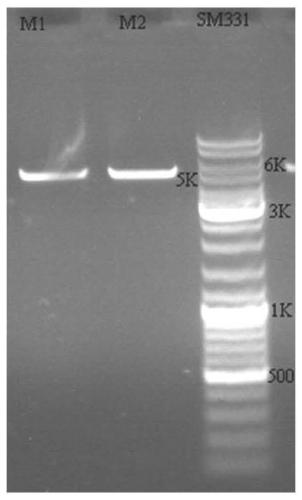
[0140] The construction of the nanobody library provided by the embodiments of the present invention is specifically:
[0141] 1) Experimental design
[0142] The M13 phage display system was selected to display the VHH antibody library, which consists of pMECS phagemid vector, E.coliTG1 and M13KO7 helper phage. In the phagemid vector pMECS, the sequence before the Pst I restriction site is the coding sequence of the pelB secretion signal peptide and some amino acids in the first framework region of the antibody. The pelB signal peptide can guide the secretion of subsequent polypeptides into the periplasmic cavity. The Not I restriction site is followed by the coding sequence of HA and 6×His tag, which can be used for purification or detection of fusion protein. The sequence immediately following it encodes the phage PIII capsid protein ( Figure 4 shown). There is an amber stop codon between the 6×His tag and the gene III sequence, and 10% to 20% of the amber stop codon ca...
Embodiment 3
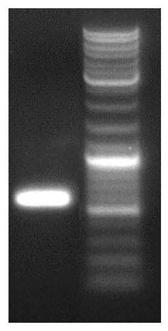
[0161] The identification and expression of proteins provided in the embodiments of the present invention are specifically:
[0162] (1) Construction of mammalian cell expression vector (plasmid template, His-Flag tag added to the C-terminus)
[0163] 1. Amplify and extract the vector plasmid containing the target gene.
[0164] 2. Subcloning into eukaryotic expression vector pcDNA3.1.
[0165] 3. Sequencing to verify the accuracy of the constructed plasmid.
[0166] 4. Obtain the recombinant plasmid pcDNA3.1 by pumping.
[0167] (2) Mammalian cell culture, protein expression and purification small test
[0168] 1. Cell lines and materials
[0169] Cell line: HEK293 cells.
[0170] Medium: DMEM (10% serum), DMEM (serum-free).
[0171] Petri dish: 10cm dish or 15cm dish
[0172] 2. HEK293 cell transfection (10cm dish)
[0173] (1) 24 hours before transfection with 4-5X10 6 The total amount of cells per 10cm culture dish is plated, and the transfection can be carried ou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com