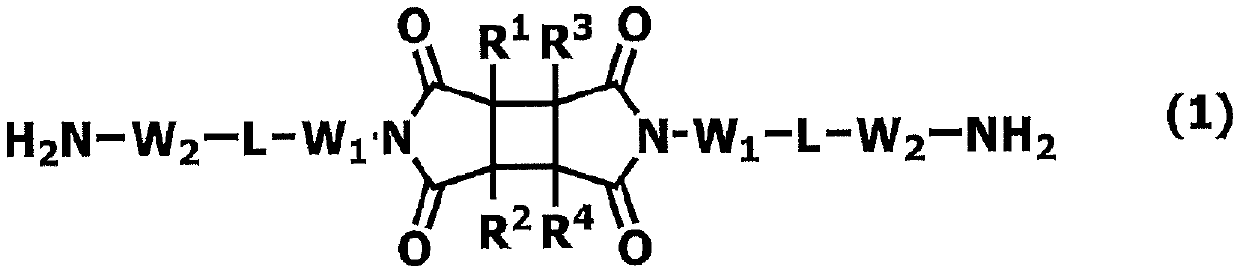
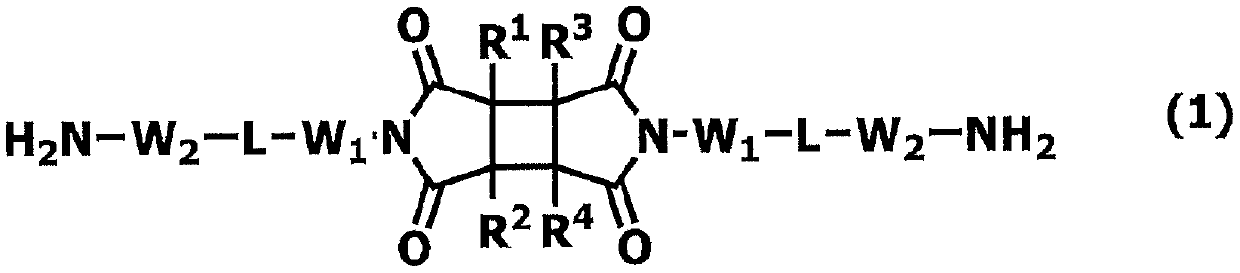
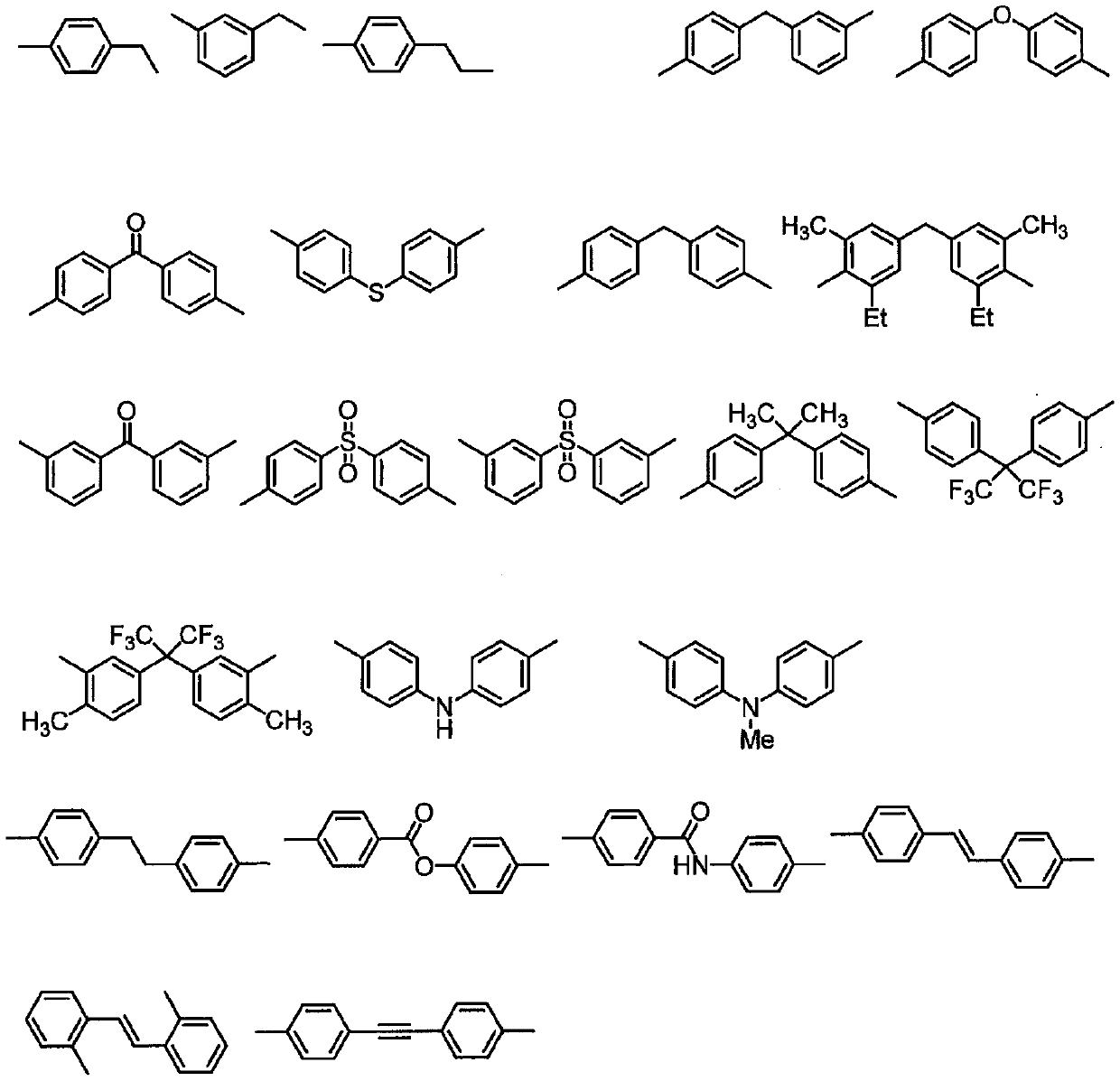
Novel polymer and diamine compound
A technology based on amine compounds and carbon numbers, which is applied in the field of new polymers and diamine compounds, can solve the problems of volume change and inability to obtain liquid crystal display characteristics, and achieve high voltage retention, high abrasion resistance, and excellent display characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0188] Examples are given below to describe the present invention in more detail, but the present invention is not limited thereto.
[0189] The structures of diamine compounds used in Examples are shown below.
[0190]
[0191]
[0192]
[0193]
[0194] DA-1 to DA-8 and DA-10 to DA-15 are novel compounds not disclosed in literature etc., and their synthesis methods will be described in detail in Synthesis Examples 1 to 14 below.
[0195] DA-9 was synthesized using the synthesis method described in the patent document (WO2017-057854).
[0196] Abbreviations of organic solvents used in Examples and the like are as follows.
[0197] NMP: N-methyl-2-pyrrolidone.
[0198] BCS: butyl cellosolve.
[0199] THF: Tetrahydrofuran.
[0200] DMF: N,N-Dimethylformamide.
[0201] CH 2 Cl 2 : Dichloromethane.
[0202] CHCl 3 : Chloroform.
[0203] MeOH: Methanol.
[0204] EtOH: ethanol.
[0205] IPA: Isopropyl Alcohol.
[0206] 1,3-DMCBDA: 1,3-Dimethyl-1,2,3,4-cyclob...
Synthetic example 1
[0212] Synthesis of [DA-1]:
[0213]
[0214] Put 4-[(4-aminophenoxy)methoxy]aniline (230.0g, 999mmol) and THF (1600g) into a 3L four-necked flask, add di-tert-butyl dicarbonate (218.0g, 999 mmol), stirred at room temperature. After completion of the reaction, the reaction liquid was concentrated, and the obtained residue was separated by silica gel column chromatography (ethyl acetate:hexane=1:1 volume ratio) to obtain 158.0 g of [DA-1-1].
[0215] [DA-1-1] (132.2 g, 400 mmol) and NMP (1300 g) were charged into a 3 L four-necked flask, and 1,3-DMCBDA (40.4 g, 180 mmol) was added to a water bath, followed by stirring at room temperature for 6 hours. Next, pyridine (85.5 g, 1081 mmol) and acetic anhydride (55.2 g, 540 mmol) were thrown into the reaction liquid, and it stirred at 60 degreeC. After completion of the reaction, the reaction system was poured into pure water (5 L), and the precipitate was filtered off. MeOH (2 L) was added to the obtained crude product, and 18...
Synthetic example 2
[0219] Synthesis of [DA-2]:
[0220]
[0221] Put 4-[3-(4-aminophenoxy)propoxy]aniline (70.0g, 271mmol) and THF (500g) into a 3L four-necked flask, add di-tert-butyl dicarbonate (59.1mmol) dropwise in a water bath g, 271 mmol), stirred at room temperature. After completion of the reaction, the reaction liquid was concentrated, and the obtained residue was separated by silica gel column chromatography (ethyl acetate:hexane=1:1 volume ratio) to obtain 46.4 g of [DA-2-1].
[0222] [DA-2-1] (46.4g, 129mmol) and NMP (460g) were put into a 3L four-necked flask, and 1,3-DMCBDA (14.5g, 65mmol) was added to the water bath, and it stirred at room temperature for 6 hours. Next, pyridine (30.7 g, 388 mmol) and acetic anhydride (19.8 g, 194 mmol) were thrown into the reaction liquid, and it stirred at 60 degreeC. After completion of the reaction, the reaction system was poured into pure water (3 L), and the precipitate was filtered off. MeOH (400 ml) was added to the obtained crude pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com